Exhibit 99.1
|
Additional Positive Data from Pivotal ASPEN Study of Brensocatib in Patients with Bronchiectasis to be Presented at the 7th World Bronchiectasis Conference
|
 |
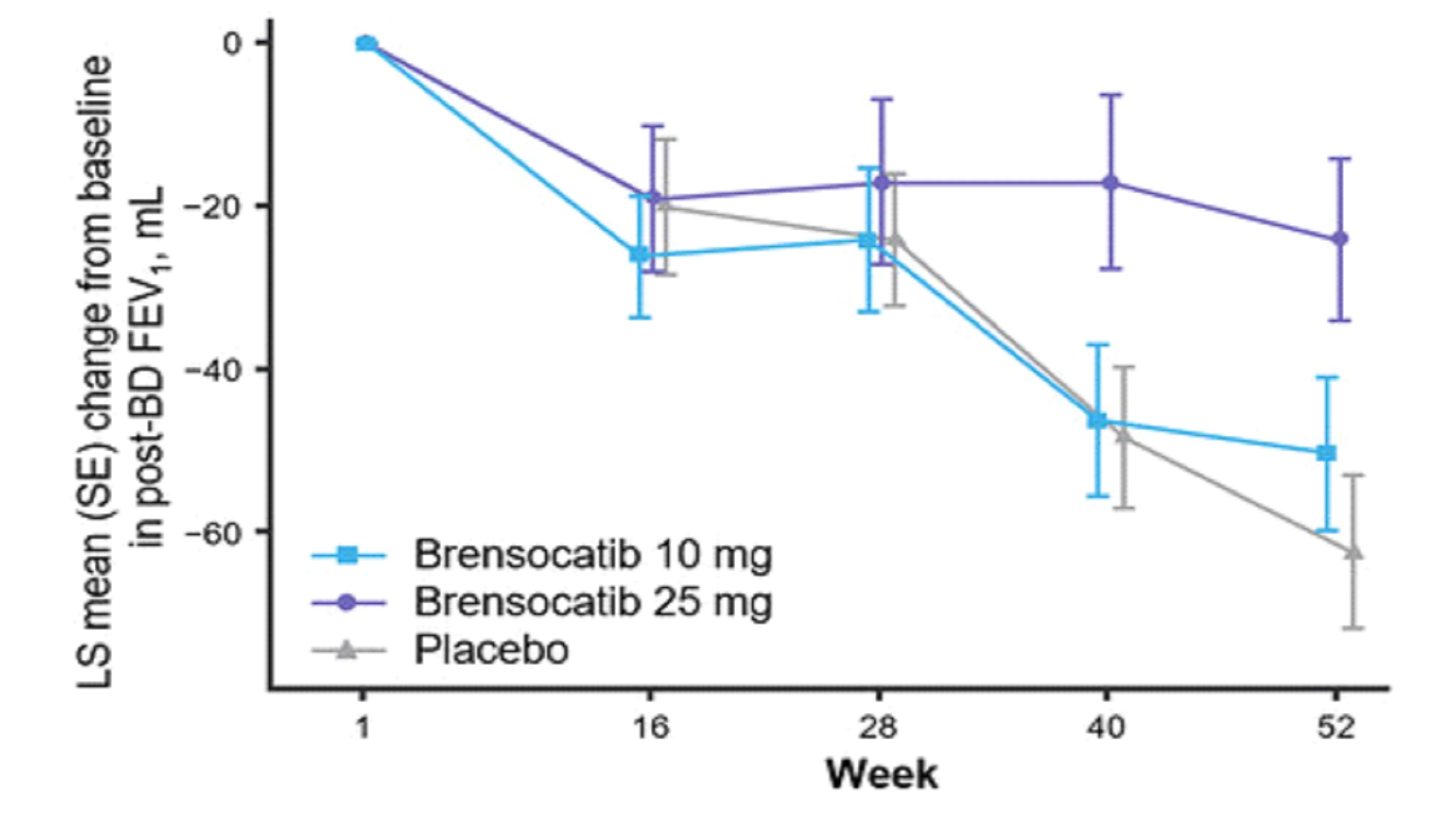
—New Graph Depicting 52-Week FEV1 Data Illustrate Significantly Less Decline in Lung Function for Brensocatib 25 mg Versus Placebo—
—Additional Exploratory Endpoints to be Presented, Including FVC and Patient-Reported BEST Score—
BRIDGEWATER, N.J., July 3, 2024 /PRNewswire/ -- Insmed Incorporated (Nasdaq: INSM), a global biopharmaceutical company on a mission to transform the lives of patients
with serious and rare diseases, today announced that additional positive results from the ASPEN study, a global, randomized, double-blind, placebo-controlled Phase 3 study to assess the efficacy, safety, and tolerability of brensocatib in patients
with non-cystic fibrosis bronchiectasis, will be presented tomorrow, July 4, 2024, at the 7th World Bronchiectasis Conference (WBC) in Dundee, Scotland. Slides from this presentation can be found here.
 |
 |
||
| Figure 1 | Figure 2 | ||
 |
 |
||
|
Figure 3
|
Figure 4
|
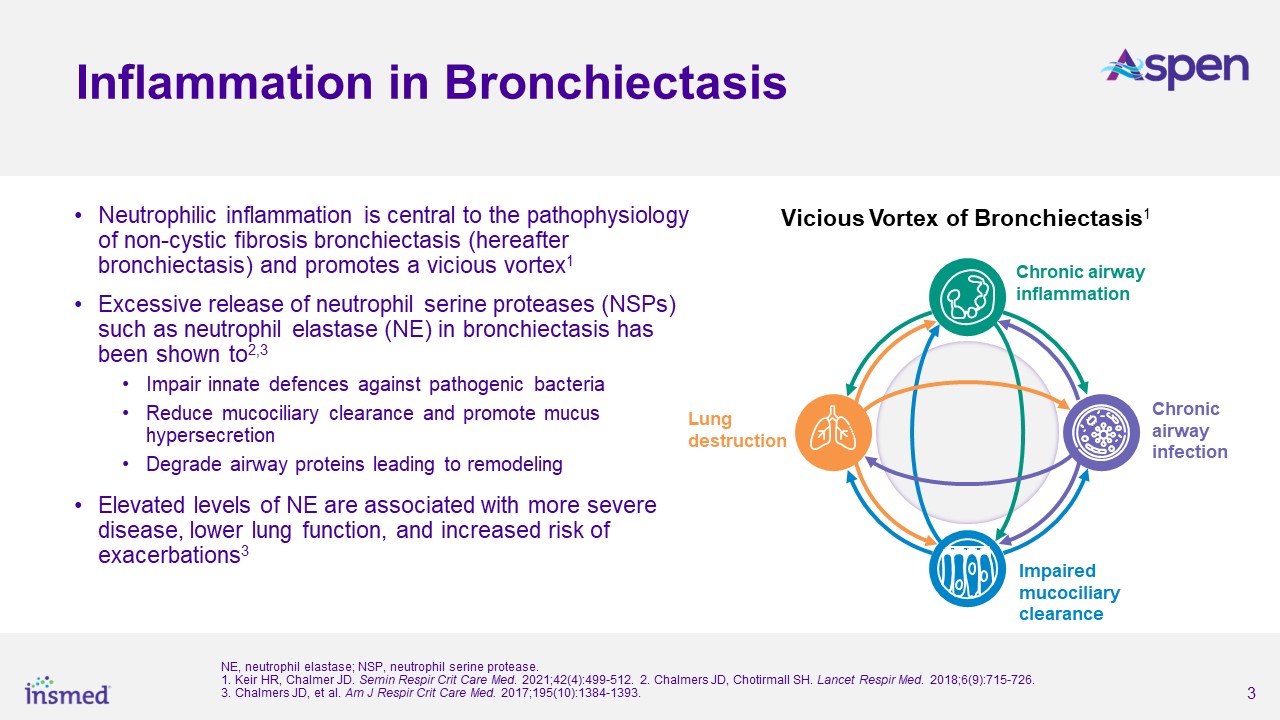
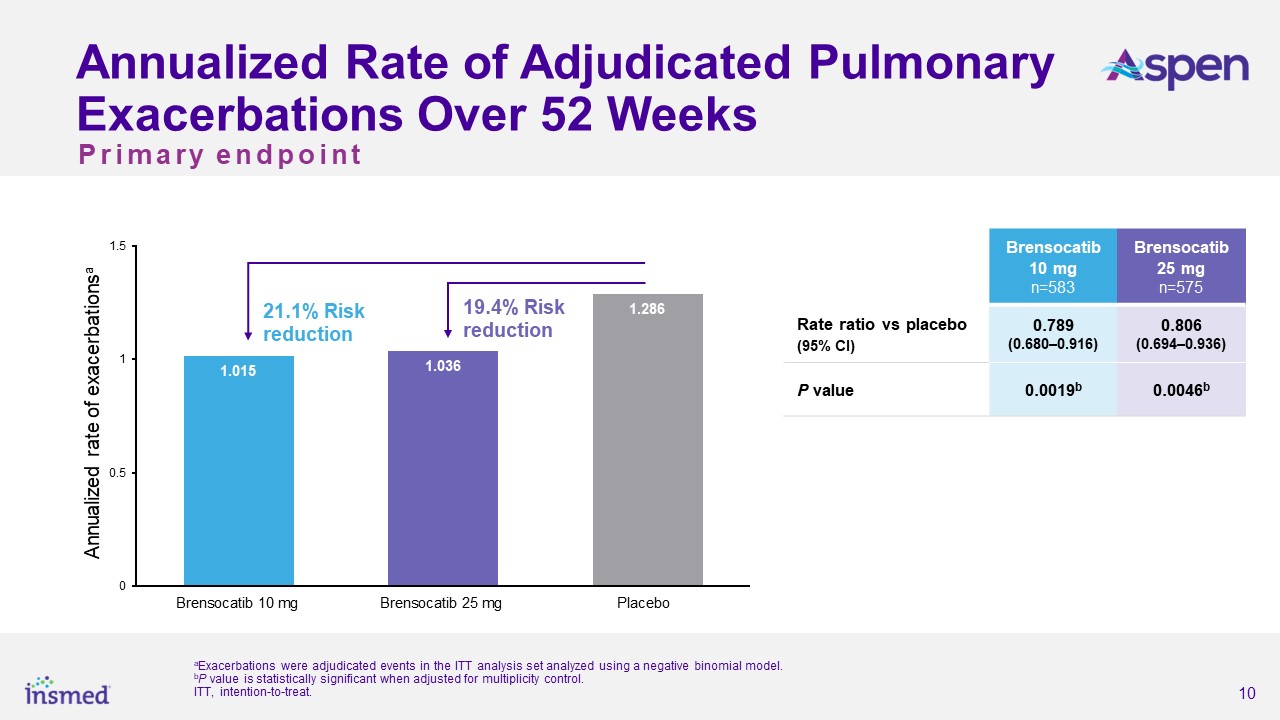
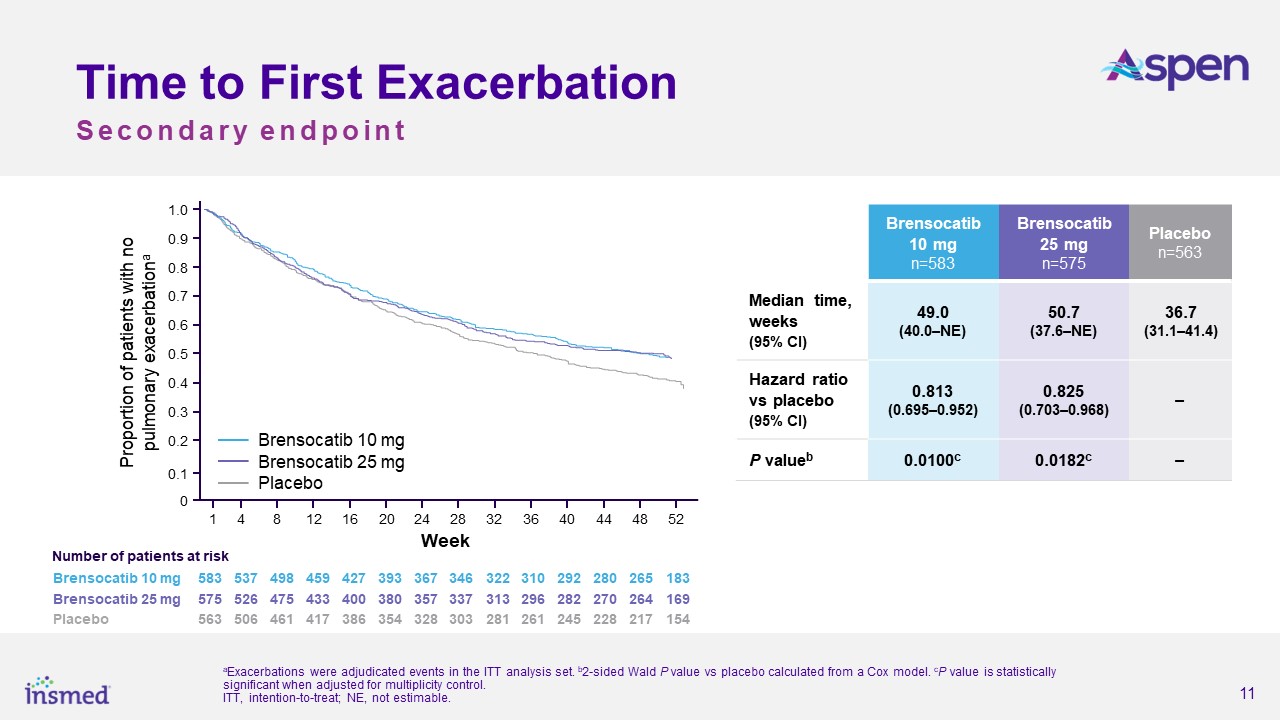
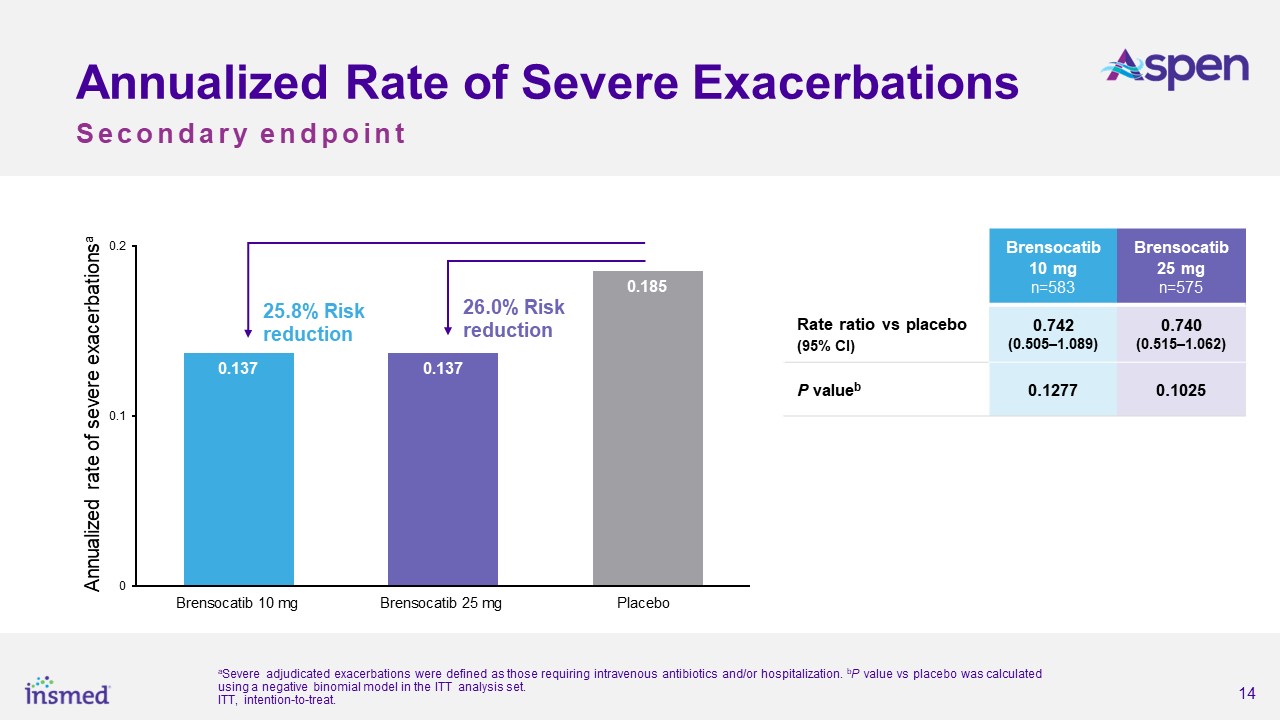
As previously announced, the ASPEN study met its primary endpoint, with both dosage strengths of brensocatib achieving statistical and clinical significance for the
reduction in the annualized rate of pulmonary exacerbations (PEs) versus placebo over the 52-week treatment period. The annualized rate of exacerbations was 1.015 for the brensocatib 10 mg group, 1.036 for the brensocatib 25 mg group, and 1.286
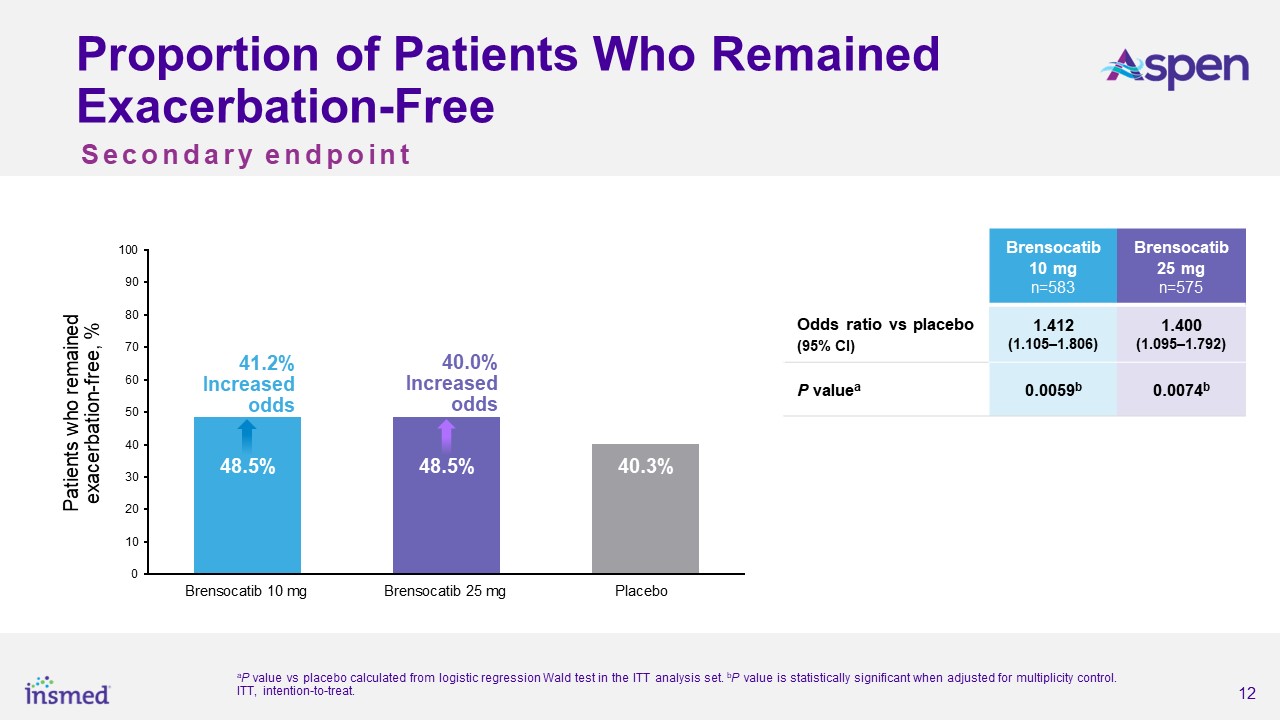
for placebo, representing a 21.1% risk reduction from placebo for the brensocatib 10 mg group (p=0.0019) and a 19.4% risk reduction for the 25 mg group (p=0.0046). Both dosage strengths of brensocatib also met several secondary endpoints,
including significantly prolonging the time to first exacerbation and significantly increasing the odds of remaining exacerbation-free over the treatment period.
“The ASPEN findings are critically important given that there is no approved treatment for bronchiectasis and there remains an urgent need for a therapy that can both
reduce pulmonary exacerbations and lessen the burden of this disease. The data announced today further underscore the positive impact brensocatib may have on patients if approved,” said lead study investigator James Chalmers, MBChB, Ph.D.,
Professor and Consultant Respiratory Physician at the School of Medicine, University of Dundee, UK. “Bronchiectasis is a progressive disease that causes patients to lose lung function over time. Therefore, I am particularly encouraged by the data
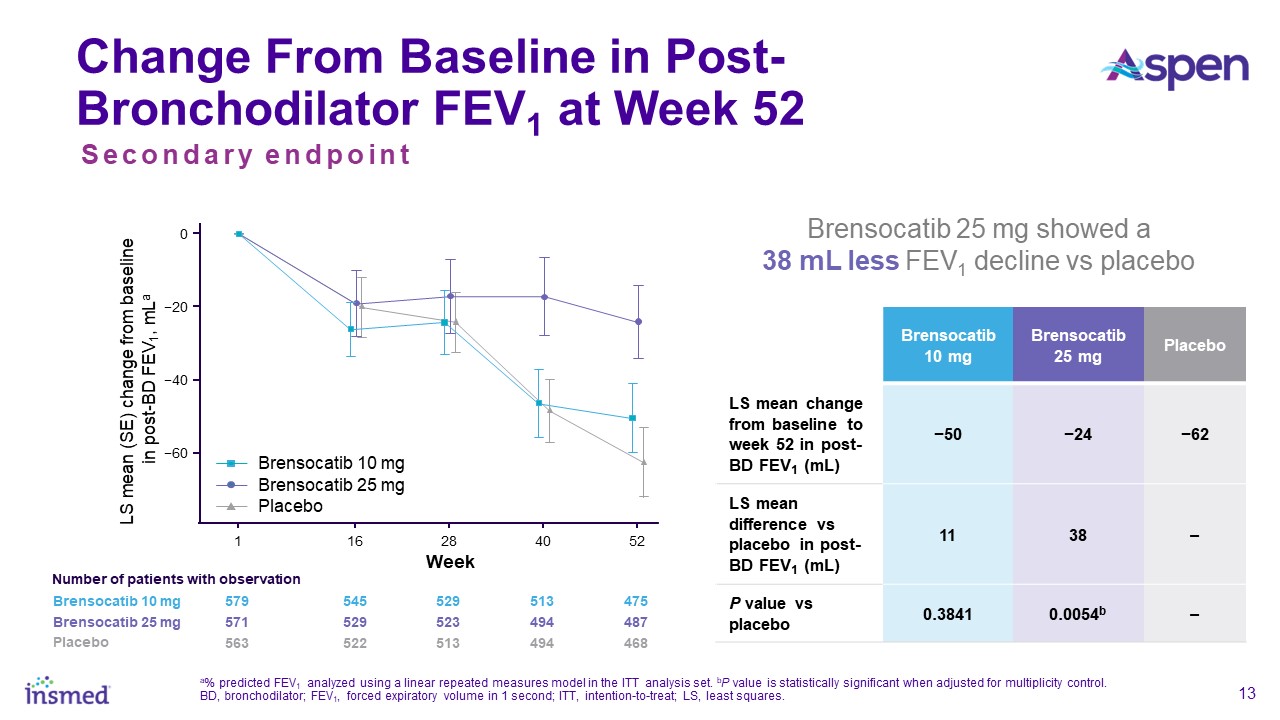
which showed that the 25 mg dose of brensocatib may slow the rate of decline of FEV1 and FVC, which represent clinically meaningful parameters of lung function that physicians consider important outcome measures.”
The study assessed change in lung function, as measured by change from baseline in post-bronchodilator forced expiratory volume over one second (FEV1) at Week 52, a key
secondary endpoint. Patients treated with brensocatib 25 mg demonstrated significantly less FEV1 decline, preserving more lung function as compared to placebo (LS mean change of 38 mL, p=0.0054). Patients in the placebo arm lost on average 62 mL of
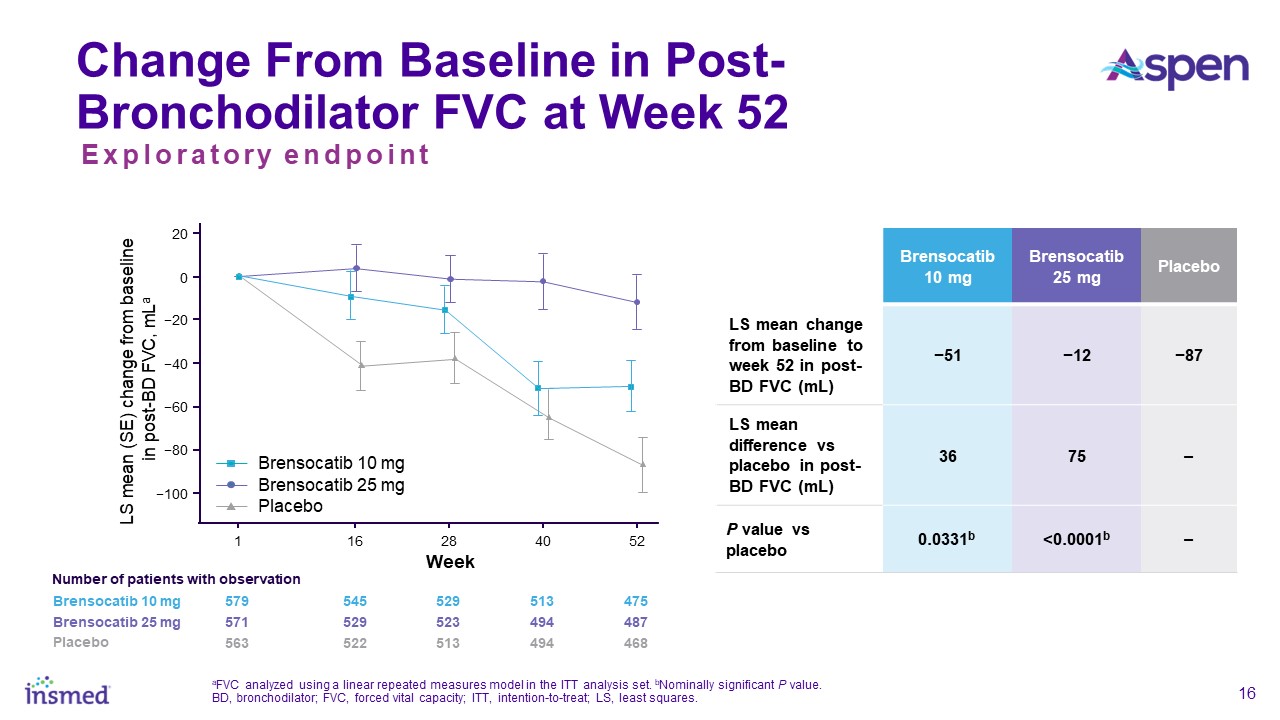
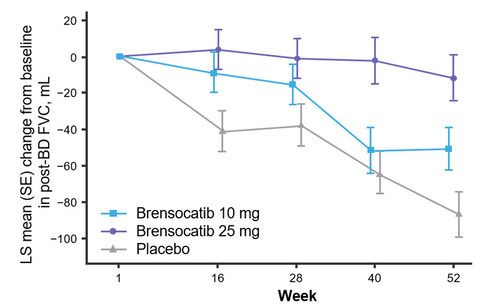
FEV1 in one year. In addition, new data will be presented at WBC measuring the change from baseline in post-bronchodilator forced vital capacity (FVC) at Week 52, another measure of lung function and an exploratory endpoint in the study. Patients
treated with brensocatib 25 mg showed nominally significantly less decline in FVC compared to placebo (LS mean change of 75 mL, p<0.0001).
“We are incredibly excited to share additional results from the ASPEN study with the bronchiectasis community at the World Bronchiectasis Conference, further building on
the positive topline results we previously shared,” said Martina Flammer, M.D., MBA, Chief Medical Officer of Insmed. “Importantly, the additional data to be presented include exploratory endpoints that further support our belief that brensocatib
may have a transformational impact on the management of bronchiectasis. The efficacy demonstrated in ASPEN, combined with a favorable safety profile that was comparable to placebo, underscore the potential for brensocatib to be used as a chronic
treatment for patients with bronchiectasis, pending approval.”
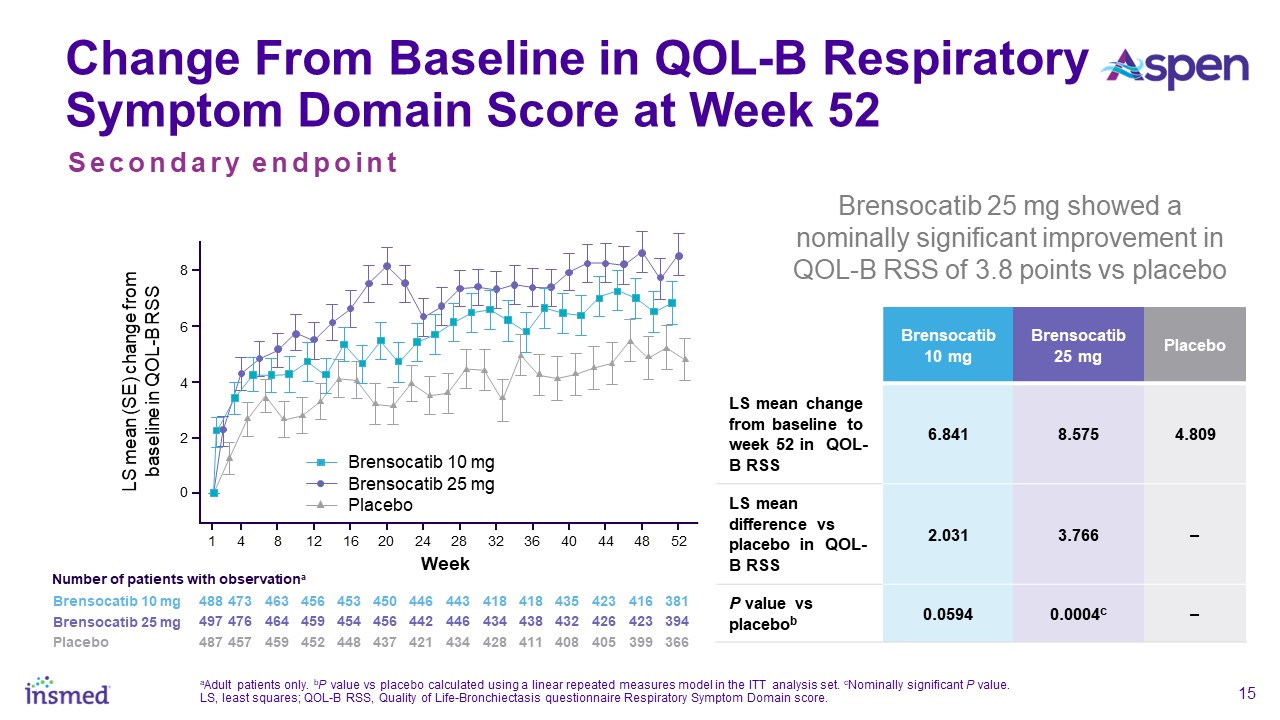
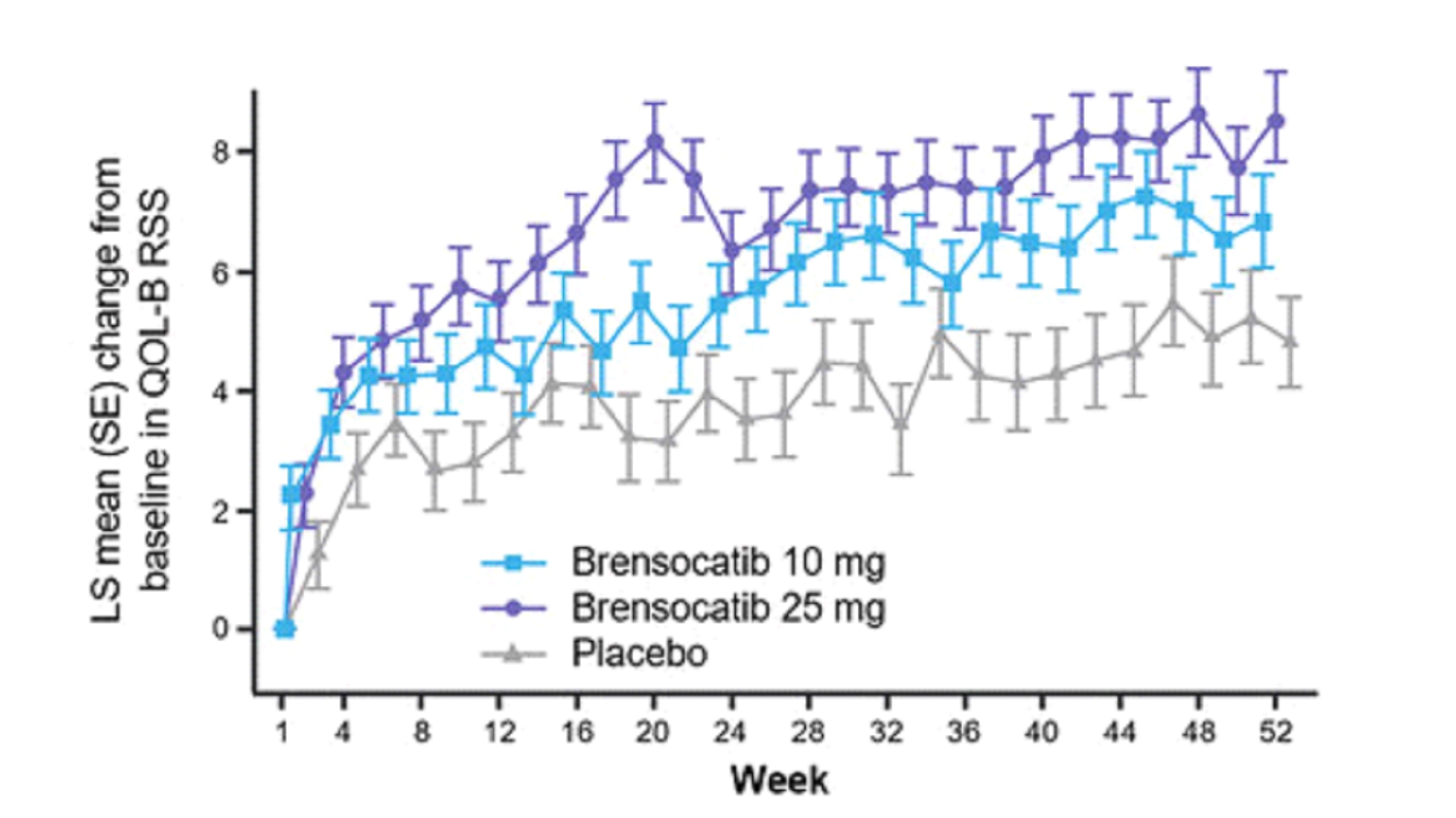
Patients in both dosage groups of brensocatib experienced numerical improvements in change from baseline in the Quality of Life-Bronchiectasis (QOL-B) Respiratory Symptom
Domain Score, with the brensocatib 25 mg dose group demonstrating a nominally significant improvement of 3.8 points versus placebo (p=0.0004). Improvements in patient reported QOL-B Respiratory Symptom Domain Score were seen as early as 4 weeks in
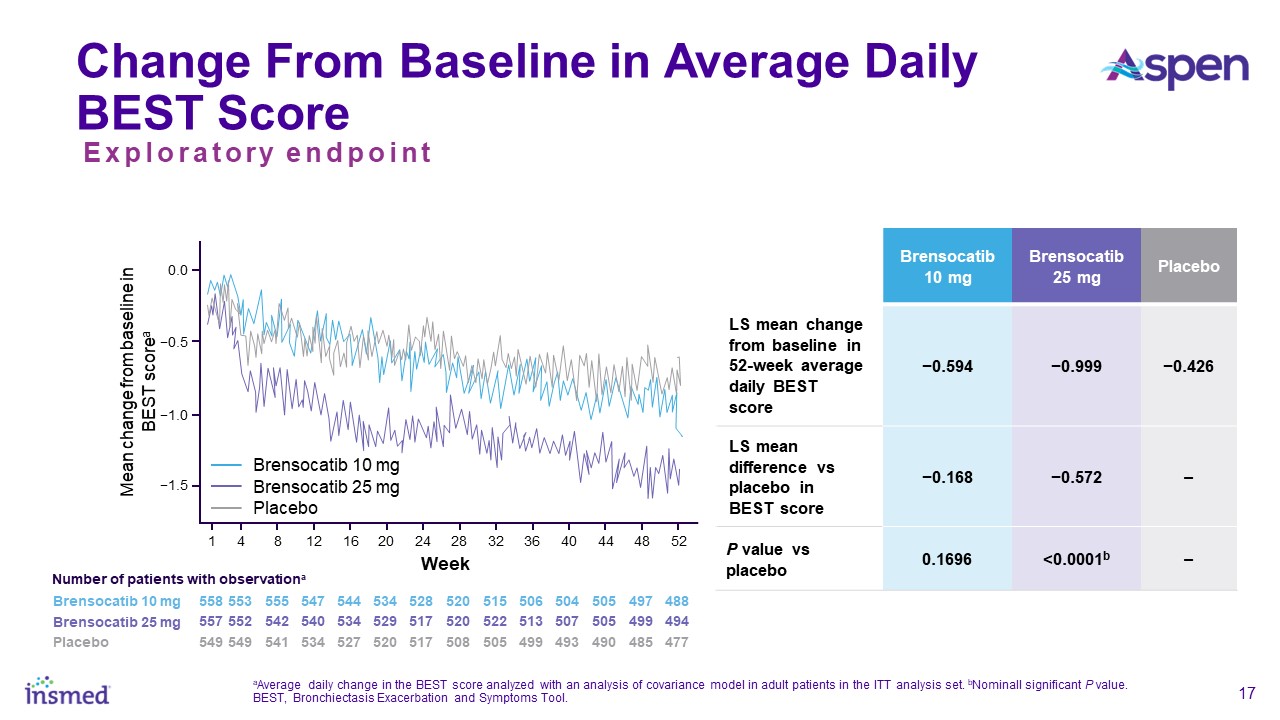
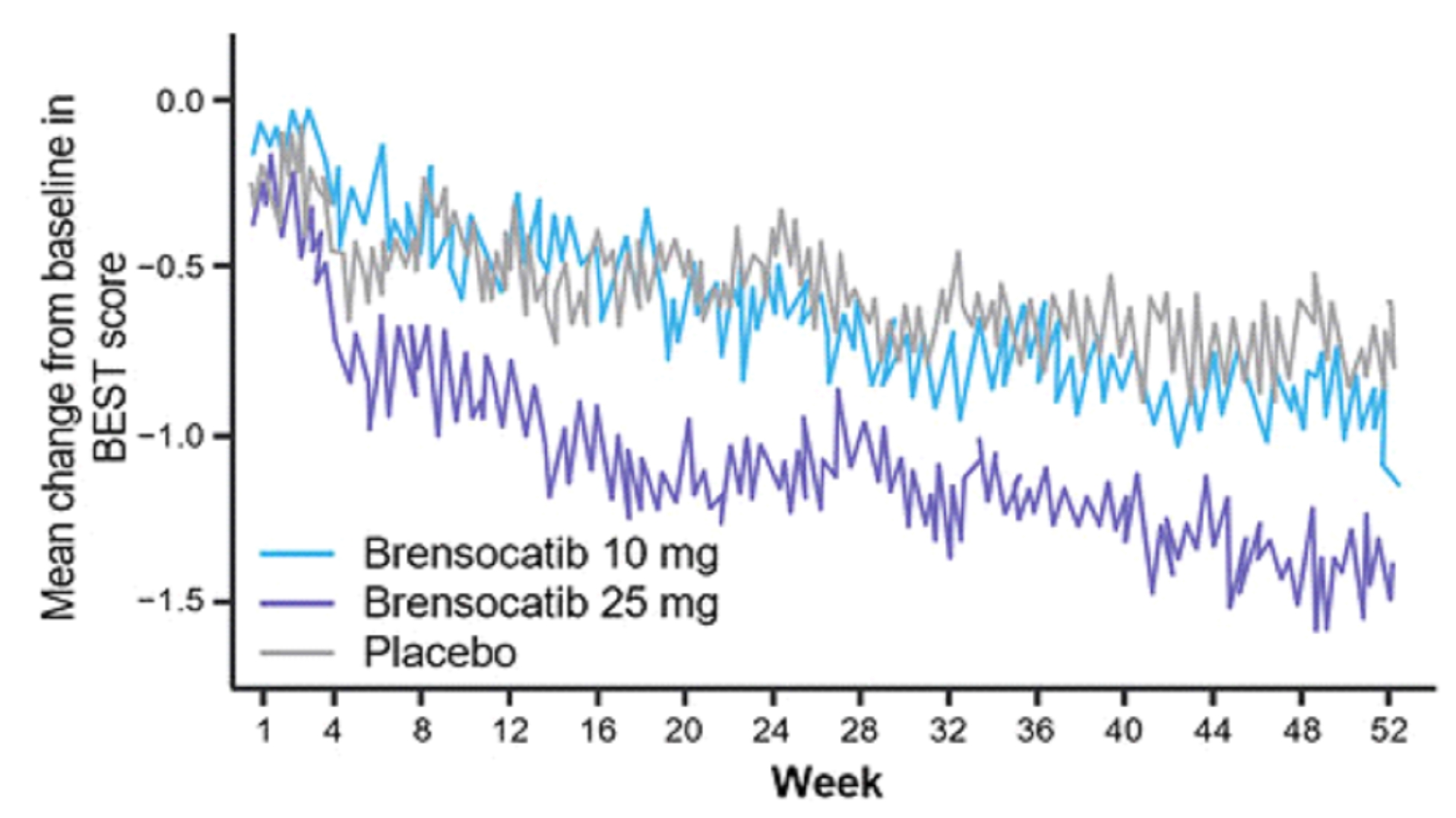
both brensocatib arms. New data will also be presented at WBC on the change in average daily bronchiectasis exacerbation and symptom tool (BEST) score, an exploratory endpoint, which is a novel symptom diary for bronchiectasis symptom burden and
detection of exacerbations. Patients treated with brensocatib 25 mg showed a nominally significant 1-point decrease in BEST score compared to placebo.
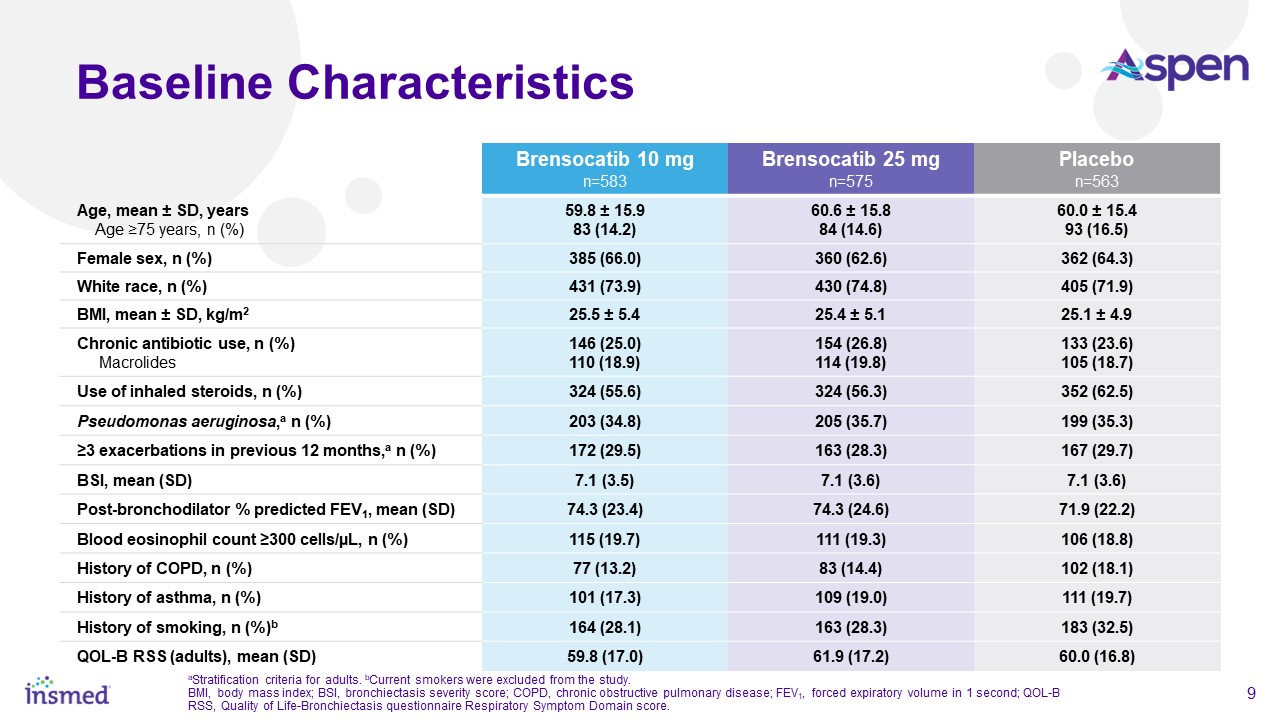
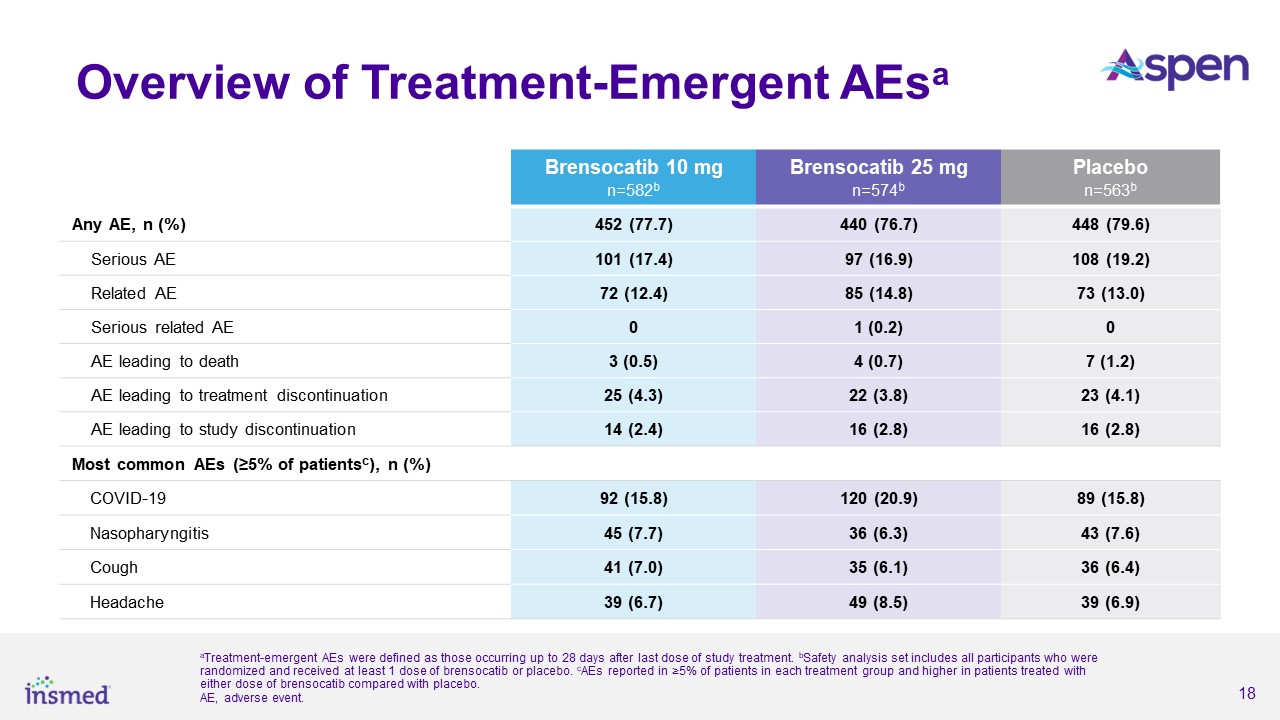
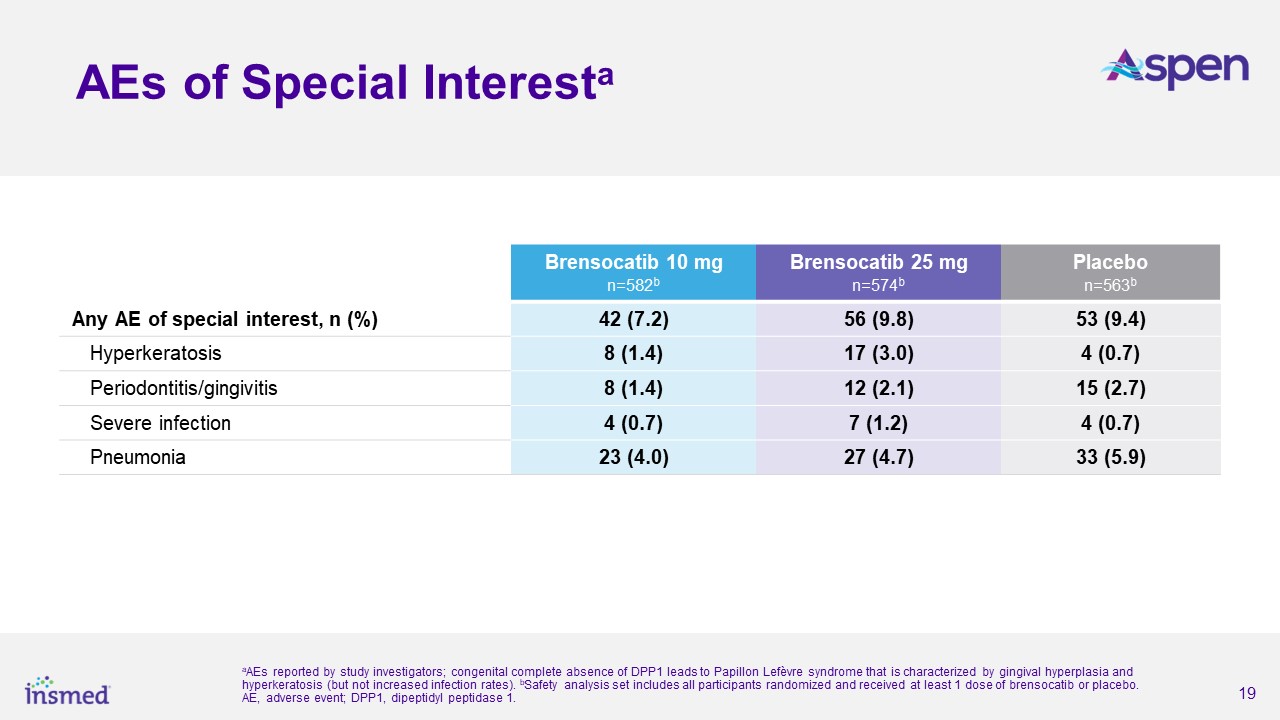
Brensocatib was well-tolerated in the study and demonstrated a favorable safety profile. Treatment-emergent adverse events (TEAEs) occurring in at least 5.0% of patients
treated with either dose of brensocatib and more frequently than in placebo were COVID-19 (15.8%, 20.9%, 15.8%), nasopharyngitis (7.7%, 6.3%, 7.6%), cough (7.0%, 6.1%, 6.4%), and headache (6.7%, 8.5%, and 6.9%) for brensocatib 10 mg, brensocatib 25
mg, and placebo, respectively.
Insmed plans to file a New Drug Application with the U.S. Food and Drug Administration for brensocatib in patients with bronchiectasis in the fourth quarter of 2024.
Pending regulatory approvals, Insmed anticipates a U.S. launch for brensocatib in mid-2025 followed by launches in Europe and Japan in the first half of 2026. If approved, brensocatib would be the first approved treatment for patients with
bronchiectasis as well as the first approved dipeptidyl peptidase 1 (DPP1) inhibitor—a new mechanism of action with the potential to address a range of neutrophil-mediated diseases.
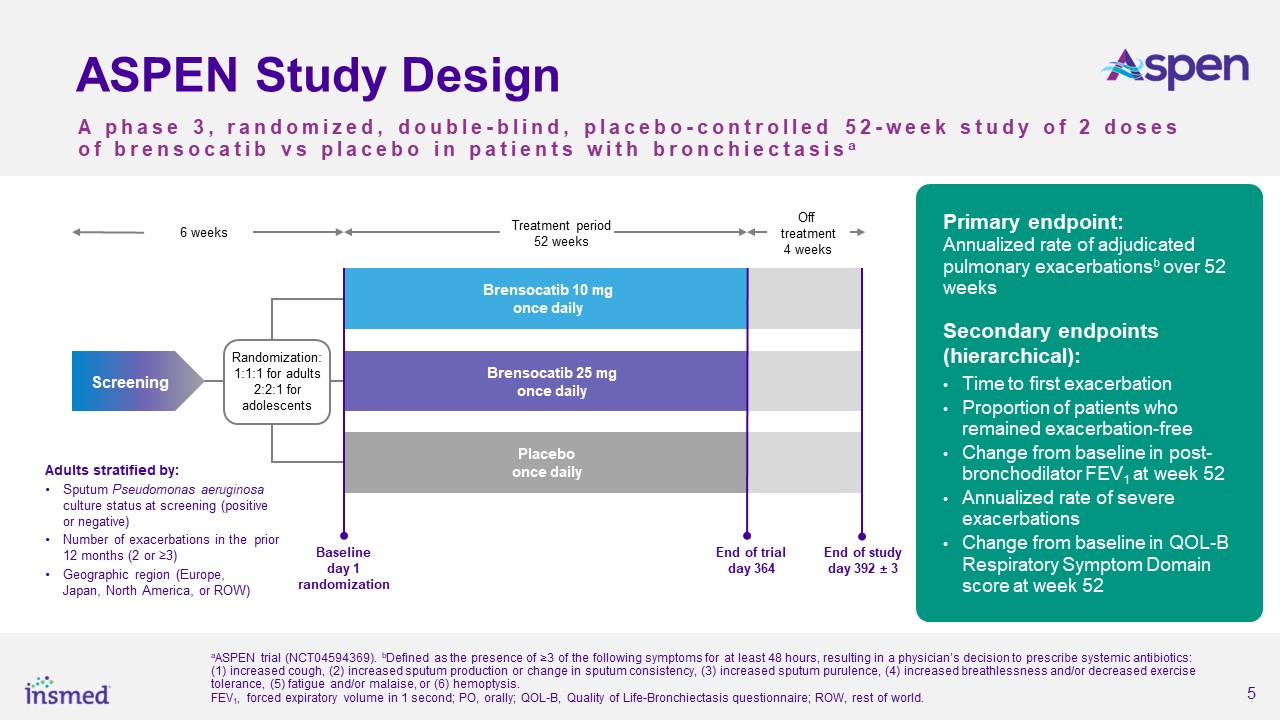
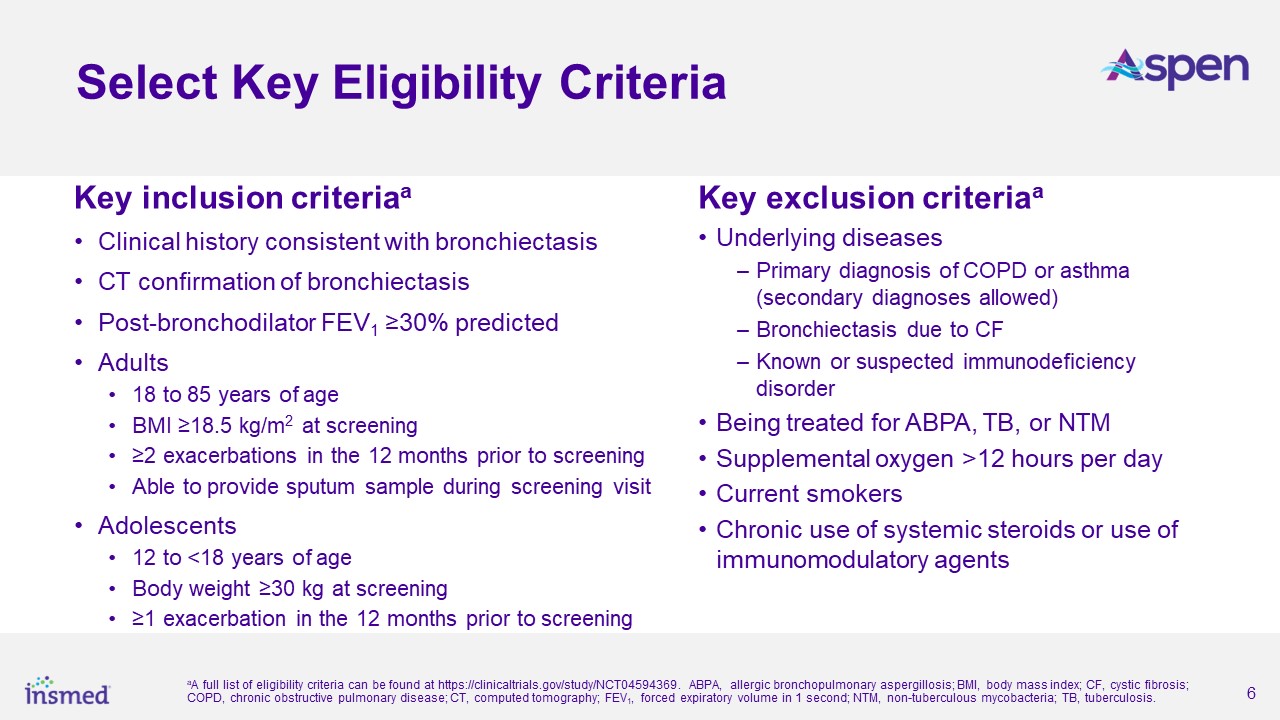
About ASPEN
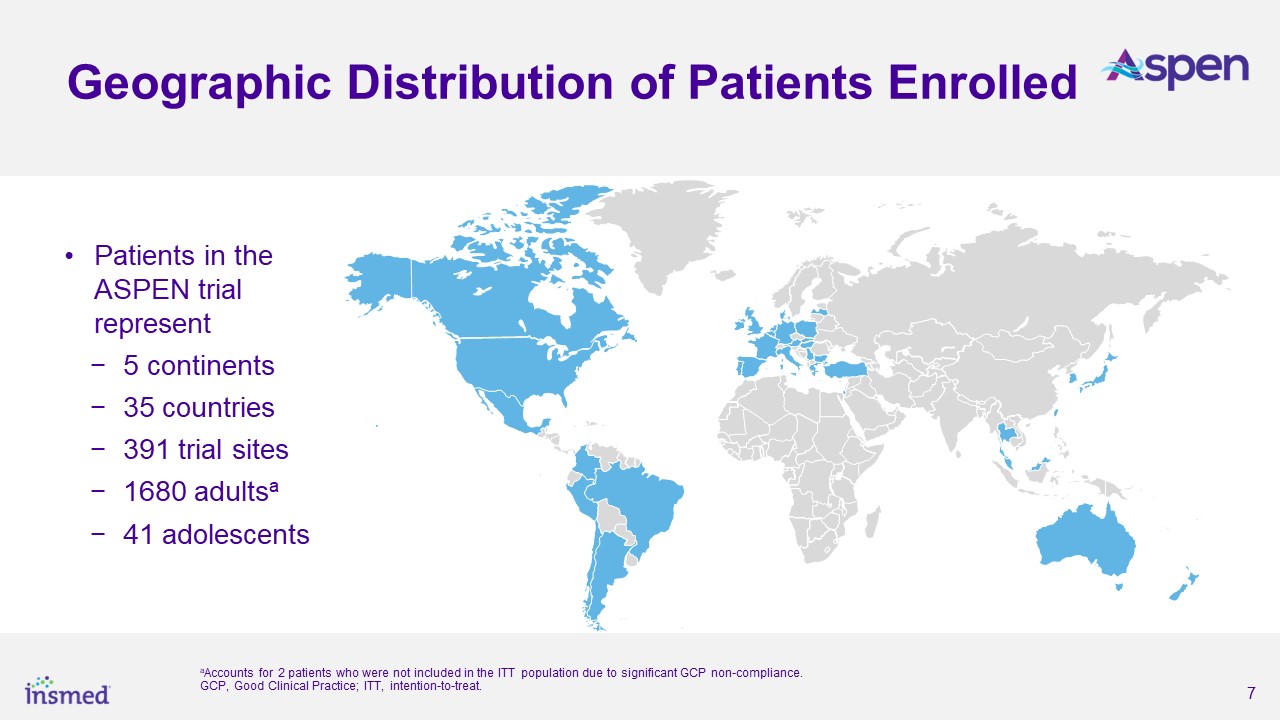
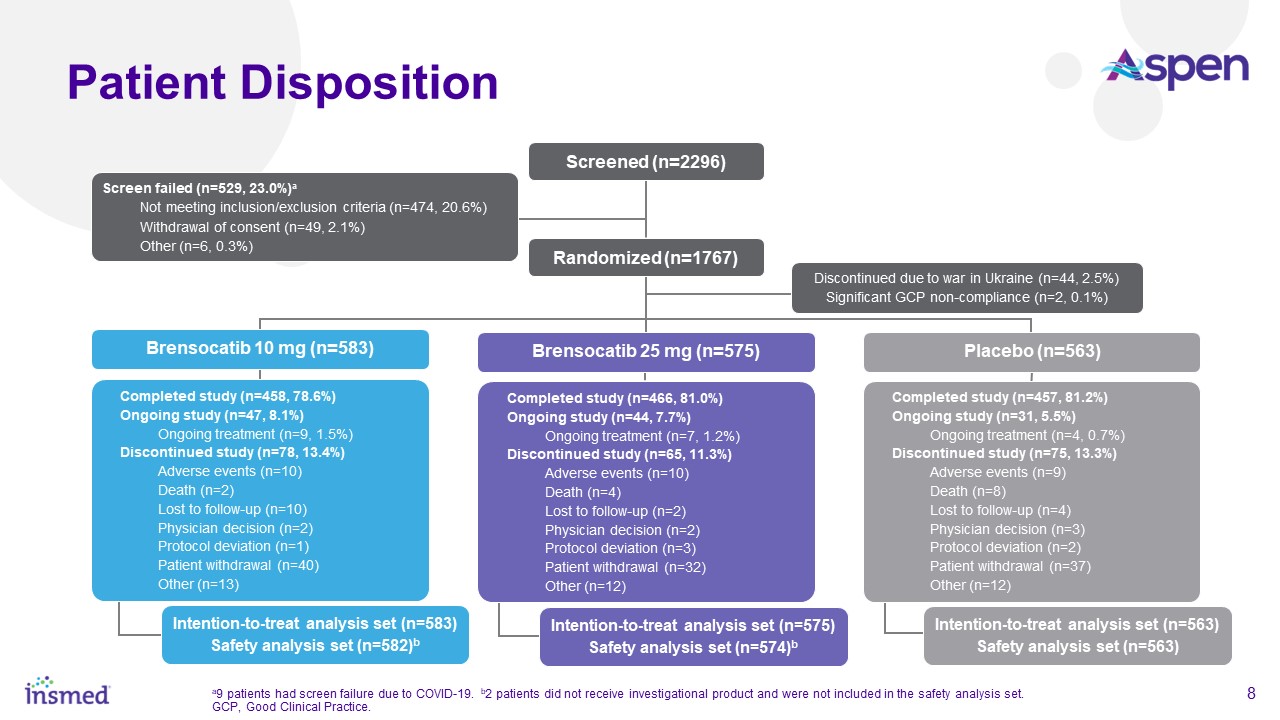
As part of the ASPEN study’s conduct, more than 460 trial sites were engaged in nearly 40 countries. After excluding sites that did not enroll any patients and all sites
in Ukraine, the total number of active sites in ASPEN was 391 sites in 35 countries. Adult patients (ages 18 to 85 years) were randomized 1:1:1 and adolescent patients (ages 12 to <18 years) were randomized 2:2:1 for treatment with brensocatib
10 mg, brensocatib 25 mg, or placebo once daily for 52 weeks, followed by 4 weeks off treatment. The primary efficacy analysis included data from 1,680 adult patients and 41 adolescent patients.
About Bronchiectasis
Bronchiectasis is a serious, chronic lung disease in which the bronchi become permanently dilated due to a cycle of infection, inflammation, and lung tissue damage. The
condition is marked by frequent pulmonary exacerbations requiring antibiotic therapy and/or hospitalizations. Symptoms include chronic cough, excessive sputum production, shortness of breath, and repeated respiratory infections, which can worsen
the underlying condition. Bronchiectasis affects approximately 500,000 patients in the U.S., 600,000 patients in Europe, and 150,000 patients in Japan, and there are currently no approved therapies specifically targeting bronchiectasis in these
regions.
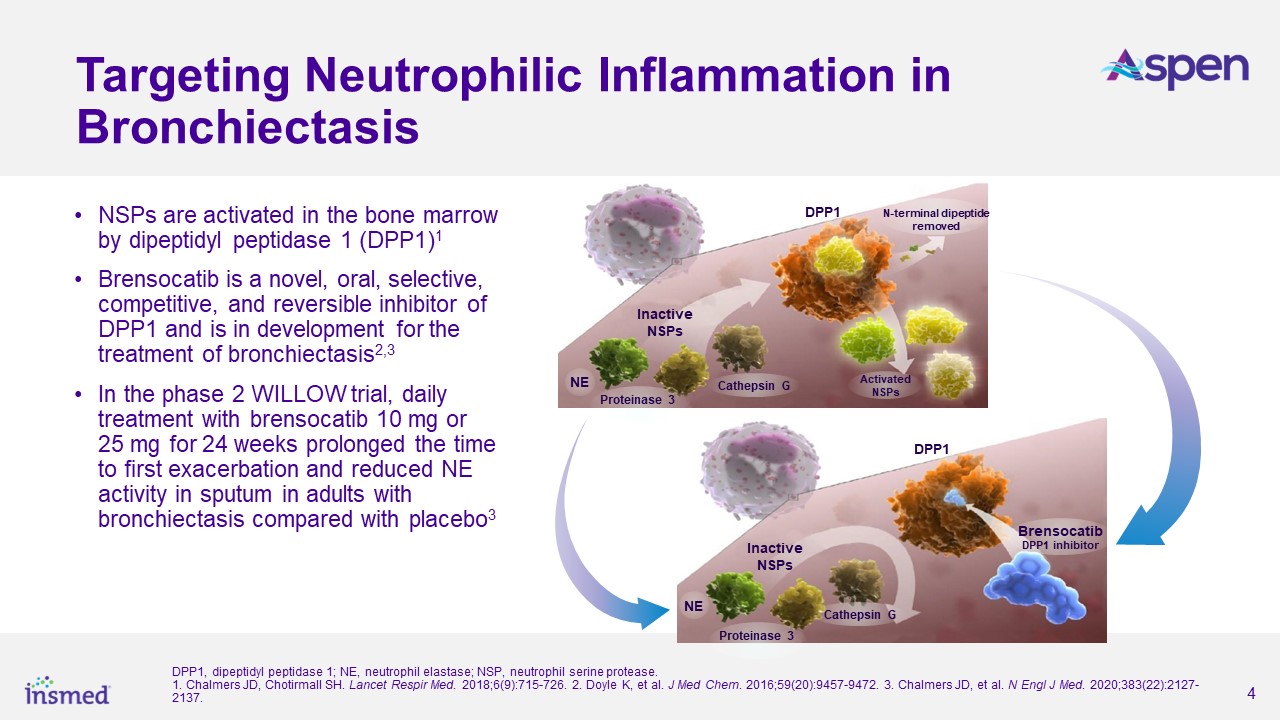
About Brensocatib
Brensocatib is a small molecule, oral, reversible inhibitor of dipeptidyl peptidase 1 (DPP1) being developed by Insmed for the treatment of patients with bronchiectasis,
CRSsNP, and other neutrophil-mediated diseases. DPP1 is an enzyme responsible for activating neutrophil serine proteases (NSPs), such as neutrophil elastase, in neutrophils when they are formed in the bone marrow. Neutrophils are the most common
type of white blood cell and play an essential role in pathogen destruction and inflammatory mediation. In chronic inflammatory lung diseases, neutrophils accumulate in the airways and result in excessive active NSPs that cause lung destruction and
inflammation. Brensocatib may decrease the damaging effects of inflammatory diseases such as bronchiectasis by inhibiting DPP1 and its activation of NSPs. Brensocatib is an investigational drug product that has not been approved for any indication
in any jurisdiction.
About Insmed
Insmed Incorporated is a global biopharmaceutical company on a mission to transform the lives of patients with serious and rare diseases. Insmed’s first commercial
product is a first-in-disease therapy approved in the United States, Europe, and Japan to treat a chronic, debilitating lung disease. The Company is progressing a robust pipeline of investigational therapies targeting areas of serious unmet need,
including neutrophil-mediated inflammatory diseases and rare pulmonary disorders. Insmed is also advancing an early-stage research engine encompassing a wide range of technologies and modalities, including artificial intelligence-driven protein
engineering, gene therapy, and protein manufacturing. Insmed is headquartered in Bridgewater, New Jersey, with additional offices and research locations throughout the United States, Europe, and Japan. Visit www.insmed.com to learn more.
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. “Forward-looking statements,” as that term is defined in the
Private Securities Litigation Reform Act of 1995, are statements that are not historical facts and involve a number of risks and uncertainties. Words herein such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,”
“believes,” “estimates,” “projects,” “predicts,” “intends,” “potential,” “continues,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) may identify forward-looking statements.
The forward-looking statements in this press release are based upon the Company’s current expectations and beliefs, and involve known and unknown risks, uncertainties and
other factors, which may cause the Company’s actual results, performance and achievements and the timing of certain events to differ materially from the results, performance, achievements or timings discussed, projected, anticipated or indicated in
any forward-looking statements. Such risks, uncertainties and other factors include, among others, the following: the risk that the full data set from the ASPEN study or data generated in further clinical trials of brensocatib will not be
consistent with the topline results of the ASPEN study or any additional results of the ASPEN study; failure to obtain, or delays in obtaining, regulatory approvals for brensocatib in the U.S., Europe or Japan; failure to successfully commercialize
brensocatib, if approved by applicable regulatory authorities, in the U.S., Europe or Japan, or to maintain U.S., European or Japanese approval for brensocatib once approved; uncertainties in the degree of market acceptance of brensocatib by
physicians, patients, third-party payors and others in the healthcare community; inaccuracies in the Company’s estimates of the size of the potential markets for brensocatib or in data the Company has used to identify physicians; expected rates of
patient uptake, duration of expected treatment, or expected patient adherence or discontinuation rates; inability of the Company, Esteve Pharmaceuticals, S.A., Thermo Fisher Scientific, Inc. or the Company’s other third-party manufacturers to
comply with regulatory requirements related to brensocatib; the Company’s inability to obtain adequate reimbursement from government or third-party payors for brensocatib or acceptable prices for brensocatib; development of unexpected safety or
efficacy concerns related to brensocatib; failure to obtain regulatory approval for potential future brensocatib indications; restrictions or other obligations imposed on us by agreements related to brensocatib, including our license agreement with
AstraZeneca AB, and failure to comply with our obligations under such agreements; failure to successfully conduct future clinical trials for brensocatib, including due to the Company’s potential inability to enroll or retain sufficient patients to
conduct and complete the trials or generate data necessary for regulatory approval, among other things; risks that the Company’s clinical studies will be delayed or that serious side effects will be identified during drug development; failure of
third parties on which the Company is dependent to manufacture sufficient quantities of brensocatib for commercial or clinical needs, to conduct the Company’s clinical trials, or to comply with the Company’s agreements or laws and regulations that
impact the Company’s business or agreements with the Company; the strength and enforceability of the Company’s intellectual property rights or the rights of third parties; the cost and potential reputational damage resulting from litigation to
which the Company may become a party, including product liability claims; changes in laws and regulations applicable to the Company’s business and failure to comply with such laws and regulations; business or economic disruptions due to
catastrophes or other events, including natural disasters or public health crises; and inability to repay the Company’s existing indebtedness and uncertainties with respect to the Company’s need and ability to access future capital.
The Company may not actually achieve the results, plans, intentions or expectations indicated by the Company’s forward-looking statements because, by their nature,
forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. For additional information about the risks and uncertainties that may affect the Company’s
business, please see the factors discussed in Item 1A, “Risk Factors,” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 and any subsequent Company filings with the Securities and Exchange Commission (SEC).
The Company cautions readers not to place undue reliance on any such forward-looking statements, which speak only as of the date of this press release. The Company
disclaims any obligation, except as specifically required by law and the rules of the SEC, to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements
may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
Contact:
Investors:
Bryan Dunn
Executive Director, Investor Relations
Insmed
(646) 812-4030
bryan.dunn@insmed.com
Executive Director, Investor Relations
Insmed
(646) 812-4030
bryan.dunn@insmed.com
Eleanor Barisser
Associate Director, Investor Relations
Insmed
(718) 594-5332
eleanor.barisser@insmed.com
Associate Director, Investor Relations
Insmed
(718) 594-5332
eleanor.barisser@insmed.com
Media:
Mandy Fahey
Executive Director, Corporate Communications
Insmed
(732) 718-3621
amanda.fahey@insmed.com
Executive Director, Corporate Communications
Insmed
(732) 718-3621
amanda.fahey@insmed.com