UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
CURRENT REPORT PURSUANT
TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date
of report (Date of earliest event reported):
(Exact Name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction of Incorporation)
(Commission File Number) |
(IRS Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
(Registrant’s Telephone Number, Including Area Code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The
|
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On May 28, 2024, InspireMD, Inc. (the “Company”) issued a press release titled “InspireMD Announces Presentation of Positive One-Year Follow-Up Results from the C-GUARDIANS U.S. Investigational Device Exemption (IDE) Clinical Trial of CGuard at LINC 2024”. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference. A copy of the presentation that the Company presented at the Leipzig Interventional Course (LINC) 2024 (“LINC 2024”) is attached hereto as Exhibit 99.2 and incorporated by reference in this Item 7.01. A copy of the presentation is also available on our website at https://www.inspiremd.com/en/investors/investor-relations/.
In accordance with General Instruction B.2 of Form 8-K, the information in this Current Report on Form 8-K that is furnished pursuant to this Item 7.01, including Exhibit 99.1, shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
On May 28, 2024, one-year follow-up results from the Company’s C-GUARDIANS Pivotal Trial of the CGuard™ Carotid Stent System were presented at LINC 2024.
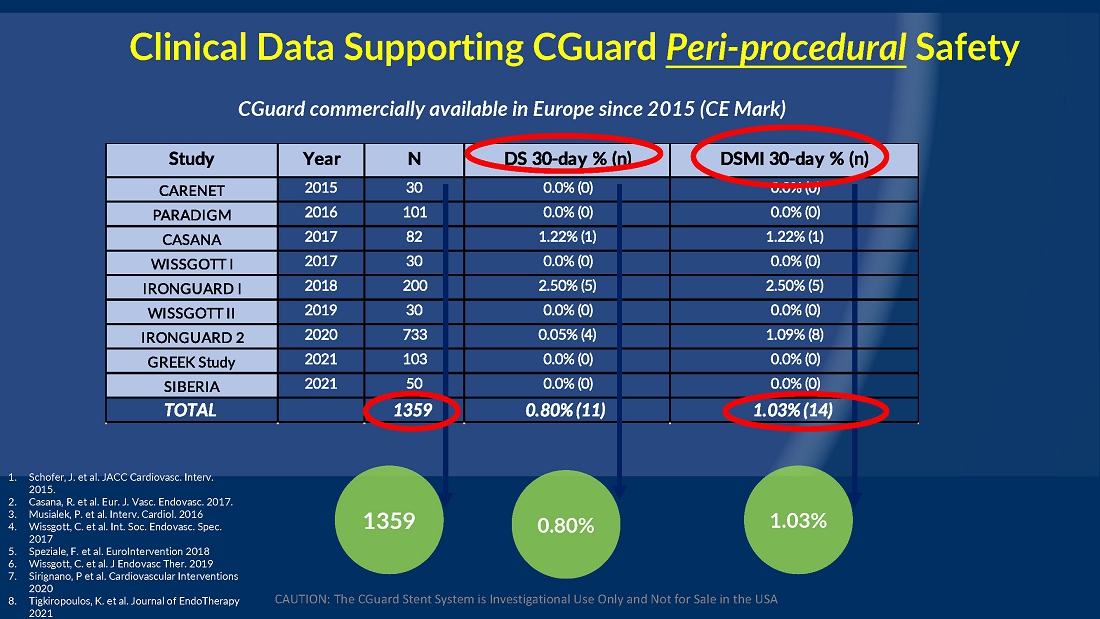
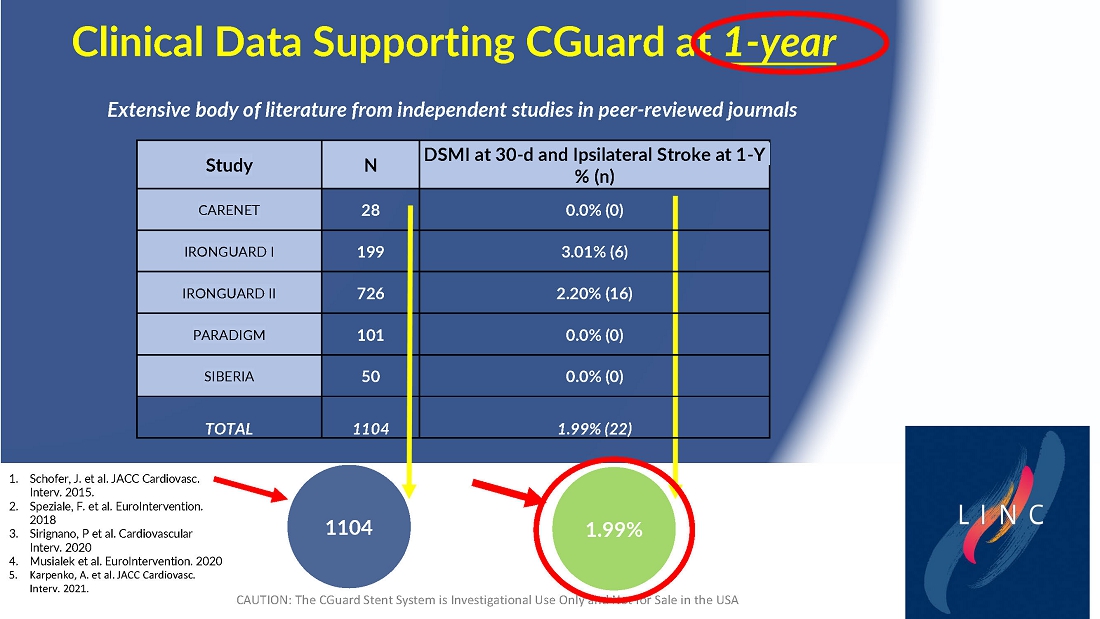
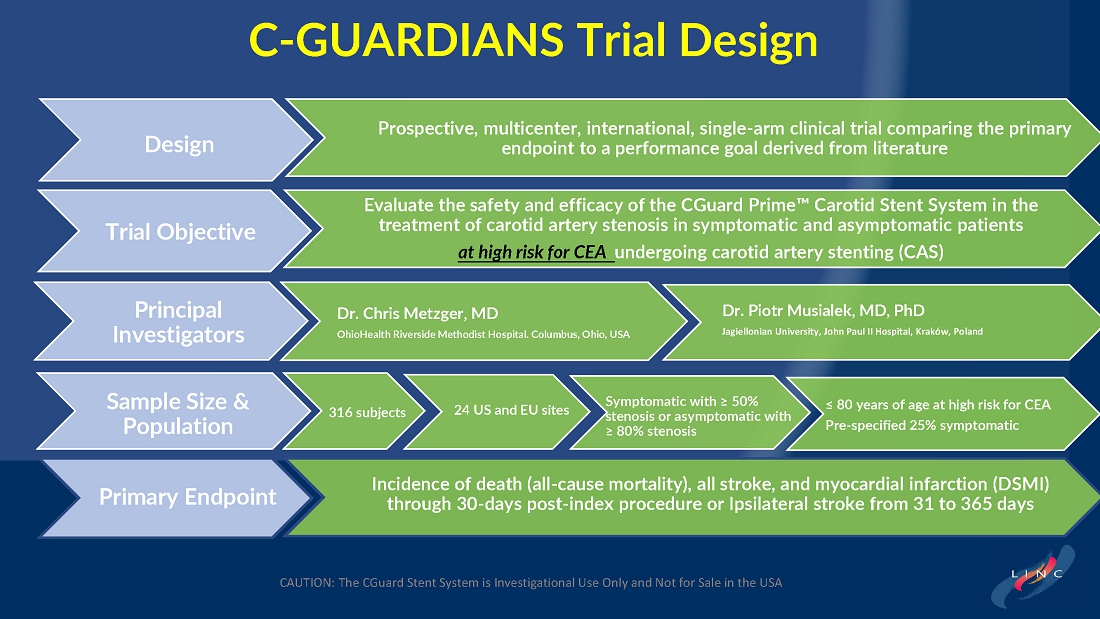
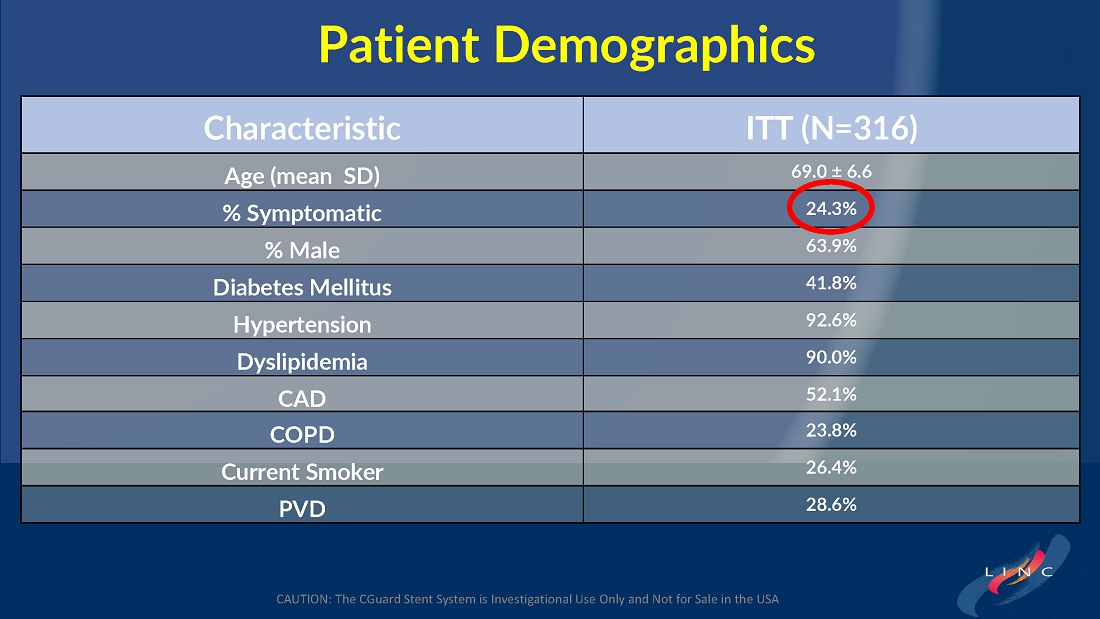
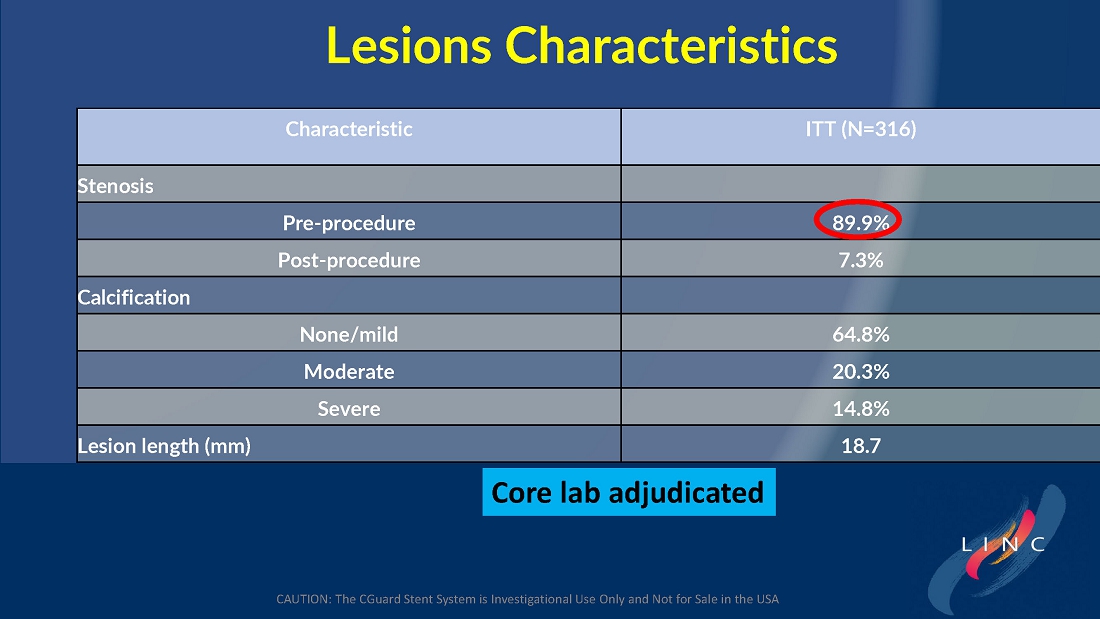
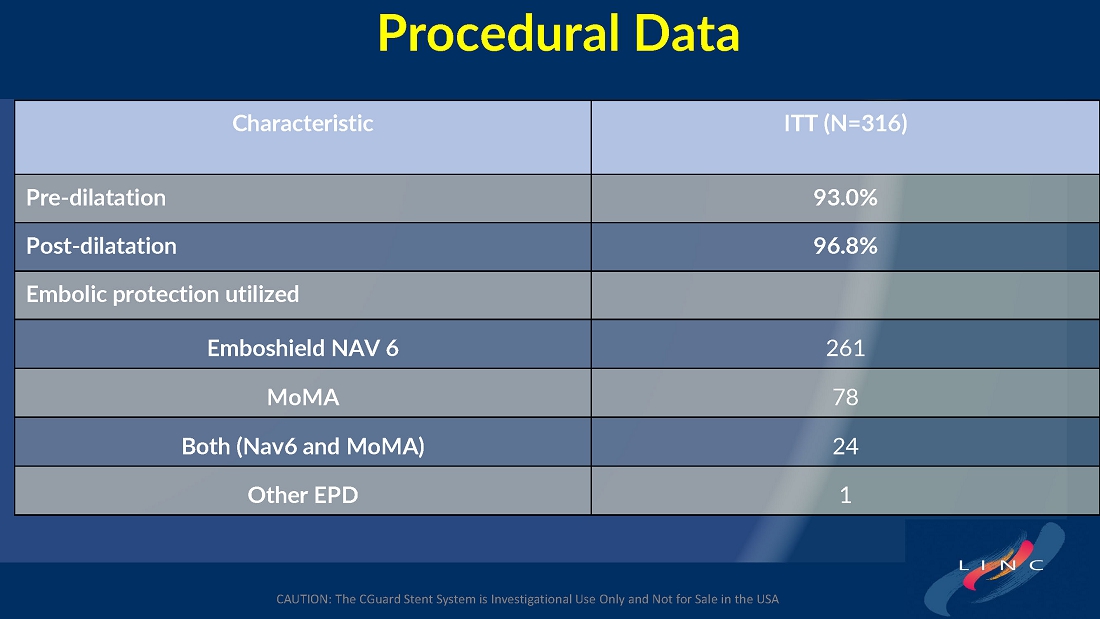
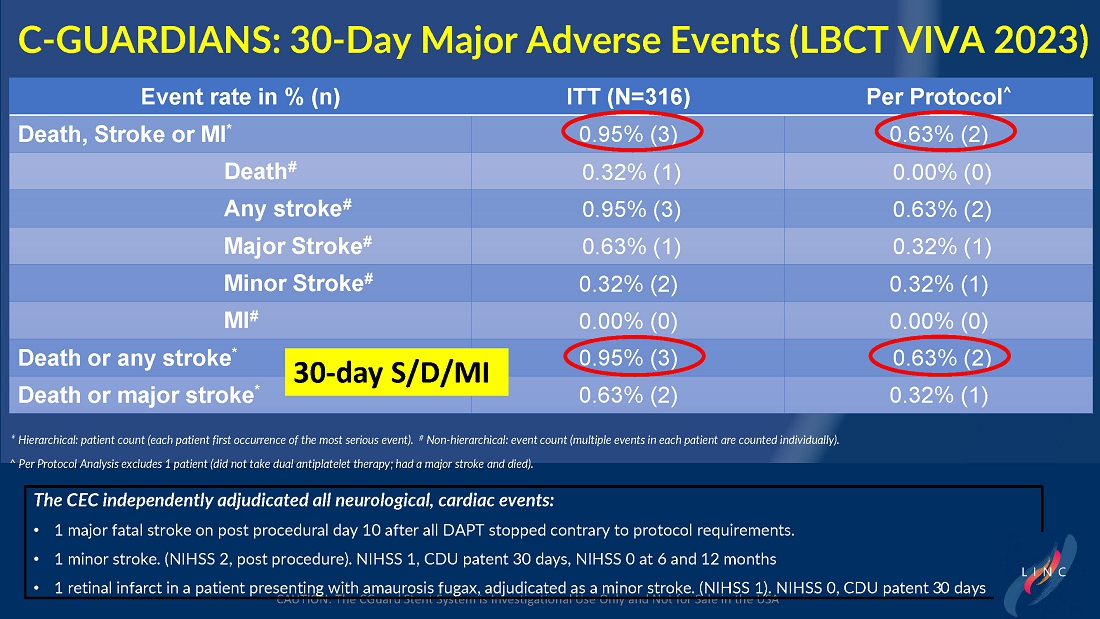
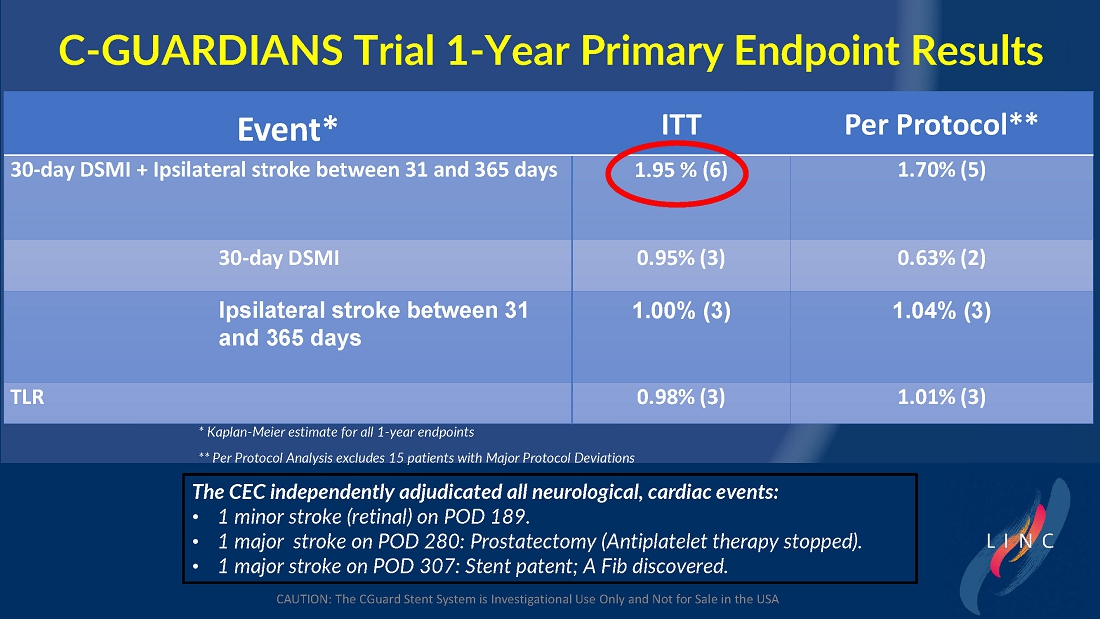
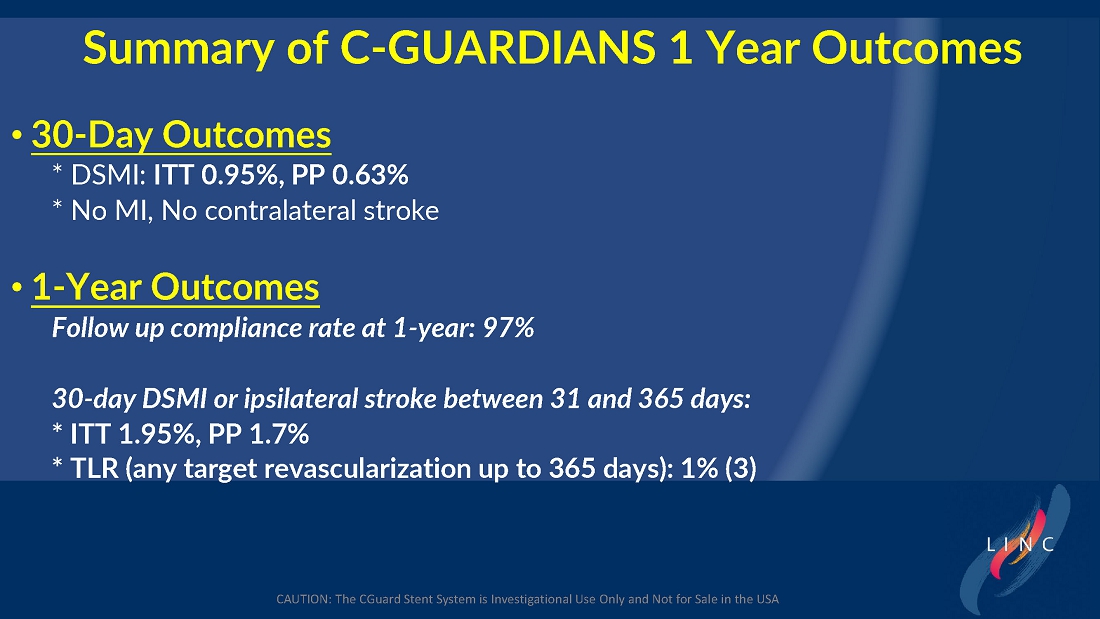
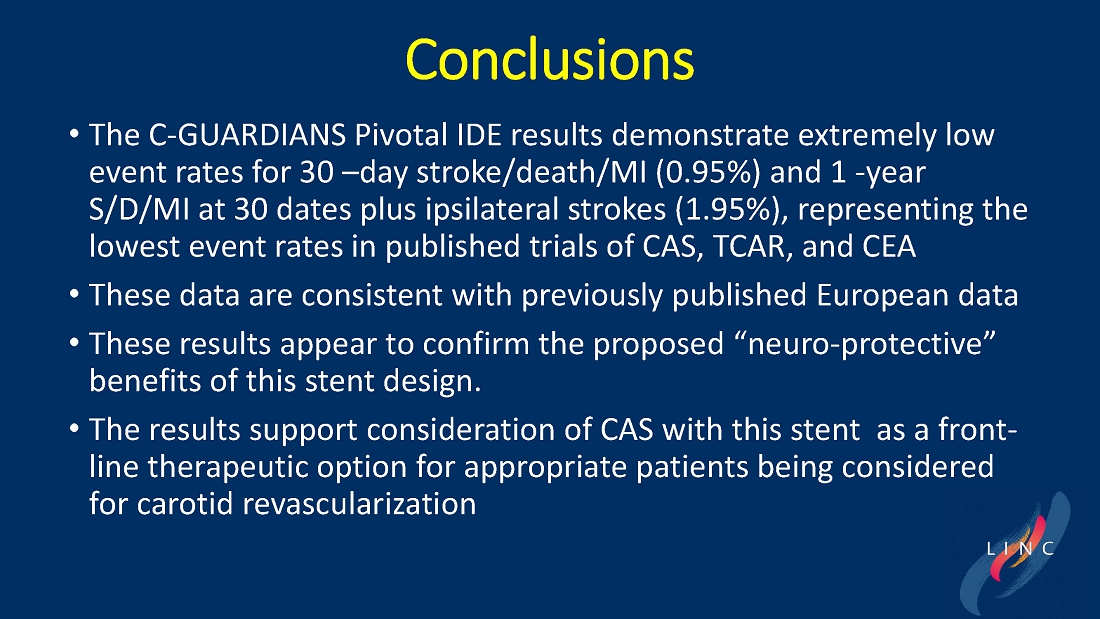
From July 2021 to June 2023, 316 patients were prospectively enrolled in a single-arm carotid artery stenting study performed at 24 sites in the United States and the European Union. The primary endpoint in the clinical trial was a composite of: (1) incidence of major adverse events including death (all-cause mortality), any stroke, or myocardial infarction (“DSMI”) through 30-days post index procedure, or (2) ipsilateral stroke from day 31 to day 365 post-procedure. Stenting with the C-Guard carotid stent system in patients with carotid artery stenosis and at high risk for carotid endarterectomy had a primary endpoint event rate (DSMI rate) of 1.95%.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit Number | Description | |
| 99.1 | Press release, dated May 28, 2024 (furnished herewith pursuant to Item 7.01) | |
| 99.2 | InspireMD LINC 2024 Presentation, May 2024 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| INSPIREMD, INC. | ||
| Date: May 28, 2024 | By: | /s/ Craig Shore |
| Name: | Craig Shore | |
| Title: | Chief Financial Officer | |
Exhibit 99.1

InspireMD Announces Presentation of Positive One-Year Follow-Up Results from the
C-GUARDIANS U.S. Investigational Device Exemption (IDE) Clinical Trial of CGuard at
LINC 2024
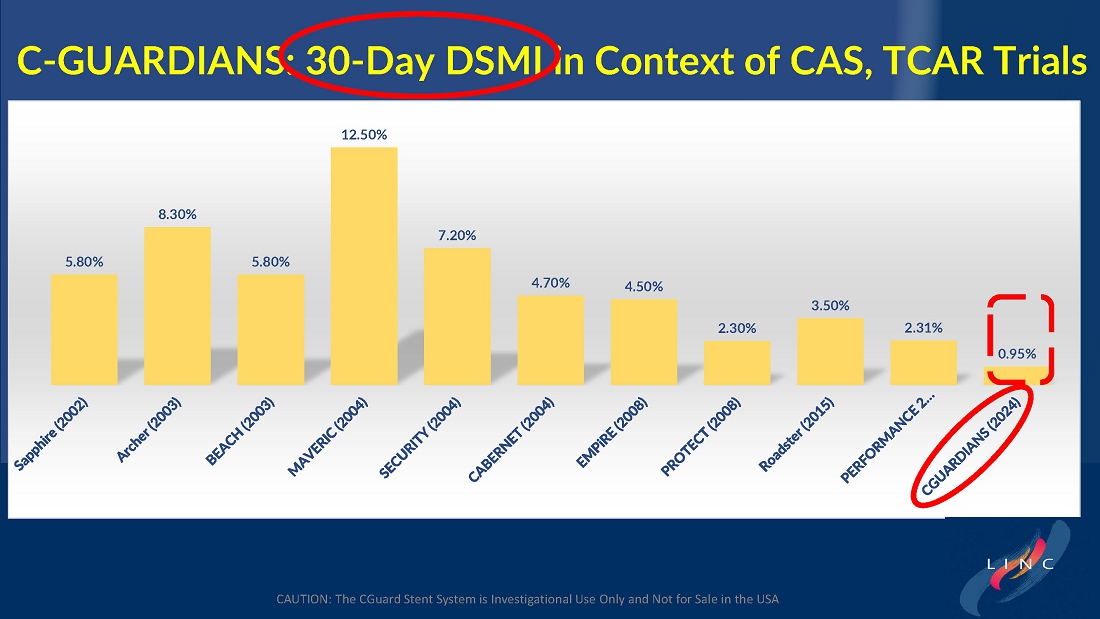
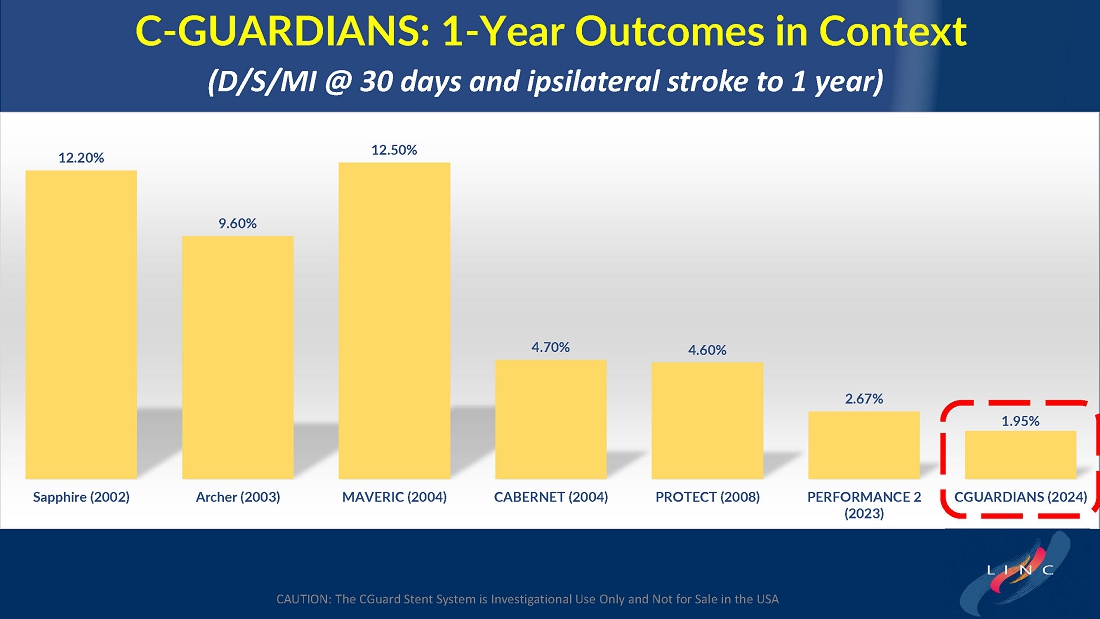
Data demonstrate lowest reported primary endpoint event rate of 1.95% through twelve months post-procedure for any carotid stent or embolic protection device pivotal trial
Study results to support a Premarket Approval (PMA) application to FDA in H2 2024
U.S. commercial launch of the CGuard™ Prime Carotid Stent System anticipated in H1 2025, if approved
Tel Aviv, Israel, and Miami, Florida — May 28, 2024 – InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard™ Embolic Prevention Stent System (EPS) for the prevention of stroke, today announced the presentation of positive one-year outcomes from its C-GUARDIANS IDE clinical trial of the CGuard™ Carotid Stent System for the treatment of carotid artery stenosis at this year’s Leipzig Interventional Course (LINC) 2024, which is being held May 28-31, in Leipzig, Germany.
Marvin Slosman, chief executive officer of InspireMD, stated, “We are very pleased to have such a significant presence at this year’s LINC conference, highlighted by a presentation of the primary endpoint results from our C-GUARDIANS clinical study. The independently adjudicated major adverse event rates through one-year are the lowest reported to date from any carotid stent or embolic protection device pivotal trial. With these data in-hand, we now have line of sight to a PMA application in the back half of this year, with preparation ongoing for a robust U.S. commercial launch in the first half of 2025, if approved. In addition to these results, we continue to be enthusiastic about our plans to introduce both CAS and TCAR solutions serving the broadest community of specialists serving the carotid revascularization market with the best implant in CGuard Prime.”
Dr. Chris Metzger, M.D., System Vascular Chief at OhioHealth, and lead investigator of the C-GUARDIANS trial, stated, “We are very excited that the one-year carefully adjudicated C-GUARDIANS data confirm the extremely low rates of stoke, death, myocardial infarction, and target vessel revascularization in this prospective trial of high-carotid endarterectomy (CEA) risk patients with obstructive carotid disease, including 25% who were symptomatic. These data confirm the potential ‘neuroprotective properties’ of this unique MicroNet technology, offering an outstanding front-line option to consider for each patient with obstructive carotid artery disease.”
Presentation details:
| Title: | One-Year Follow-Up Results from the C-GUARDIANS Pivotal Trial of the CGuard™ Carotid Stent System |
| Presenter: | Dr. D. Christopher Metzger, System Vascular Chief, OhioHealth |
| Date/time: | Tuesday, May 28th at 2:53 pm CEST (8:53am EDT) |

Presentation Highlights:
| ● | From July 2021 to June 2023, 316 patients were prospectively enrolled in this single-arm carotid artery stenting study performed at 24 sites in the US and the EU. |
| ● | The primary endpoint is a composite of: (1) incidence of major adverse events including death (all-cause mortality), any stroke, or myocardial infarction (DSMI) through 30-days post index procedure, or (2) ipsilateral stroke from day 31 to day 365 post-procedure. |
| ● | Stenting with the CGuard carotid stent system in patients with carotid artery stenosis and at high risk for carotid endarterectomy had a primary endpoint event rate of 1.95%, from procedure through 1-year follow-up. |
| ● | The presentation is available on our website at: Clinical Presentations - InspireMD |
About LINC
LINC, the Leipzig Interventional Course, is strongly committed to contributing to a systematic scientific evaluation and interdisciplinary discussion of new methods in the field of vascular medicine, allowing conclusions for daily interventional practice. LINC is an interdisciplinary live course, designed to provide a global platform, permitting the discussion of the “vascular patients” by integrating colleagues of different specialties from around the world who are performing endovascular interventions.
For more information, please visit: https://www.leipzig-interventional-course.com/
About C-GUARDIANS
The C-GUARDIANS clinical trial evaluated the safety and efficacy of the CGuard™ Carotid Stent System for the treatment of carotid artery stenosis. The study enrolled 316 patients across 24 trial sites in the U.S. and Europe.
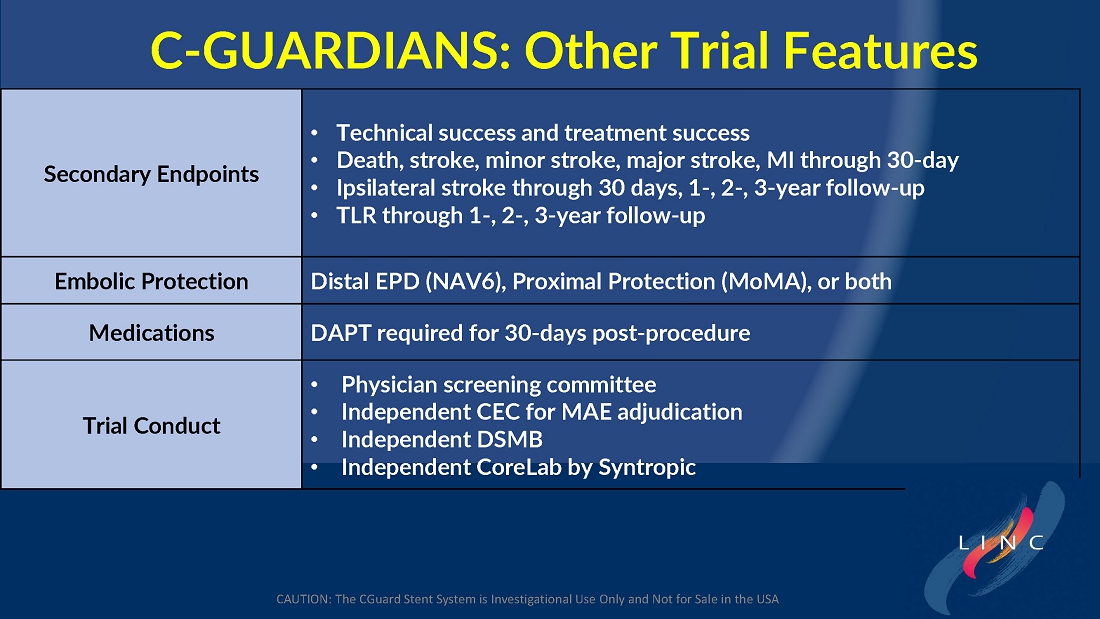
The trial included both symptomatic and asymptomatic patients undergoing carotid artery stenting (CAS). The primary endpoint includes the composite of the following: incidence of the following major adverse events: death (all- cause mortality), all stroke, or myocardial infarction (DSMI) through 30-days post-index procedure, or ipsilateral stroke from 31-365-day follow-up, based on the Clinical Events Committee (CEC) independent adjudication. The performance goal will be considered to have been met if the upper bound of the two-sided 95% confidence interval calculated from the observed primary endpoint rate is <11.6% and the p-value is <0.025.
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet® technology to make its products the industry standard for carotid stenting by providing outstanding acute results and durable, stroke-free long-term outcomes. InspireMD’s common stock is quoted on the Nasdaq under the ticker symbol NSPR.
We routinely post information that may be important to investors on our website. For more information, please visit www.inspiremd.com.

Forward-looking Statements
This press release contains “forward-looking statements.” Forward-looking statements include, but are not limited to, statements regarding InspireMD or its management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential”, “scheduled” or similar words. Examples of such statements include, but are not limited to, statements relating to the C-Guardians U.S. IDE clinical trial, including one-year results from such trial presented at LINC 2024, as well as the timing and outcome of any subsequent results, PMA or potential launch. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with our history of recurring losses and negative cash flows from operating activities, significant future commitments and the uncertainty regarding the adequacy of our liquidity to pursue our complete business objectives, and substantial doubt regarding our ability to continue as a going concern; our need to raise additional capital to meet our business requirements in the future and such capital raising may be costly or difficult to obtain and could dilute out stockholders’ ownership interests; market acceptance of our products; an inability to secure and maintain regulatory approvals for the sale of our products; negative clinical trial results or lengthy product delays in key markets; our ability to maintain compliance with the Nasdaq listing standards; our ability to generate revenues from our products and obtain and maintain regulatory approvals for our products; our ability to adequately protect our intellectual property; our dependence on a single manufacturing facility and our ability to comply with stringent manufacturing quality standards and to increase production as necessary; the risk that the data collected from our current and planned clinical trials may not be sufficient to demonstrate that our technology is an attractive alternative to other procedures and products; intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do; entry of new competitors and products and potential technological obsolescence of our products; inability to carry out research, development and commercialization plans; loss of a key customer or supplier; technical problems with our research and products and potential product liability claims; product malfunctions; price increases for supplies and components; insufficient or inadequate reimbursement by governmental and other third-party payers for our products; our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful; adverse federal, state and local government regulation, in the United States, Europe or Israel and other foreign jurisdictions; the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction; the escalation of hostilities in Israel, which could impair our ability to manufacture our products; and current or future unfavorable economic and market conditions and adverse developments with respect to financial institutions and associated liquidity risk. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
Craig Shore
Chief Financial Officer
InspireMD, Inc.
888-776-6804
craigs@inspiremd.com
Chuck Padala, Managing Director
LifeSci Advisors
646-627-8390
chuck@lifesciadvisors.com
investor-relations@inspiremd.com
Exhibit 99.2