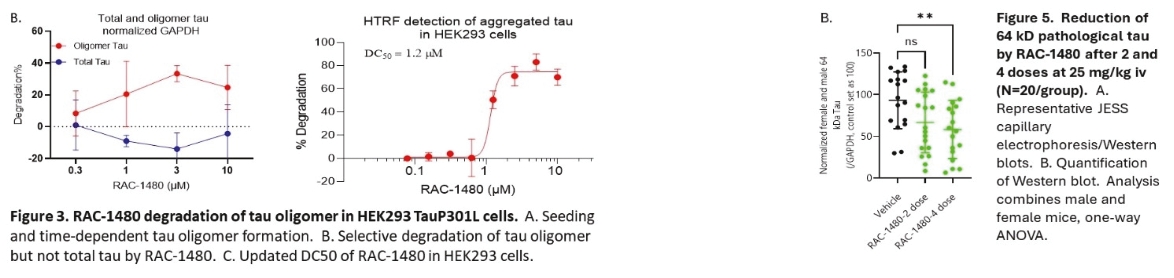
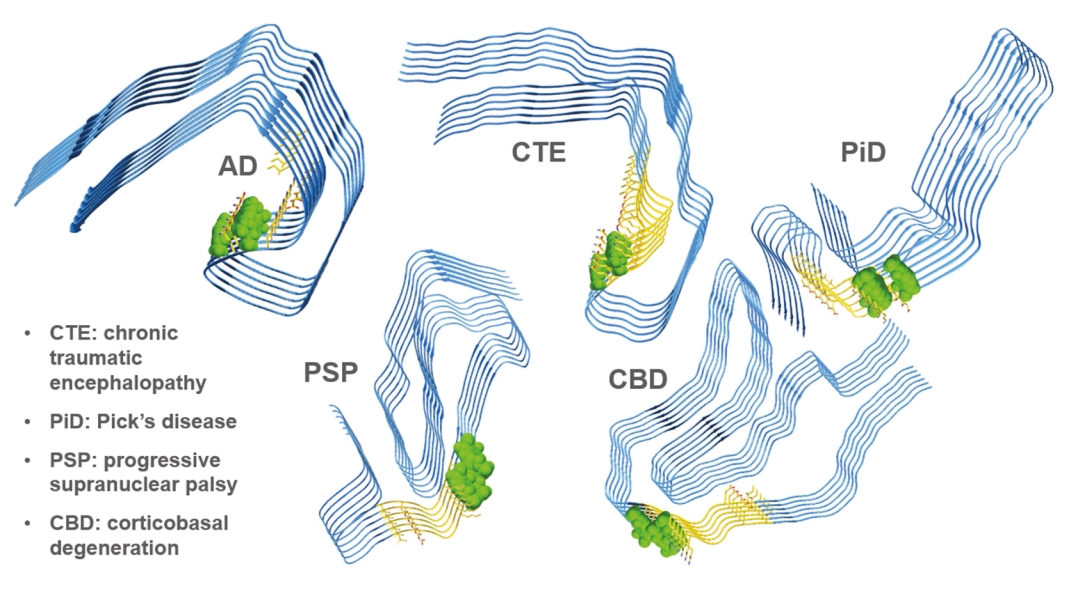
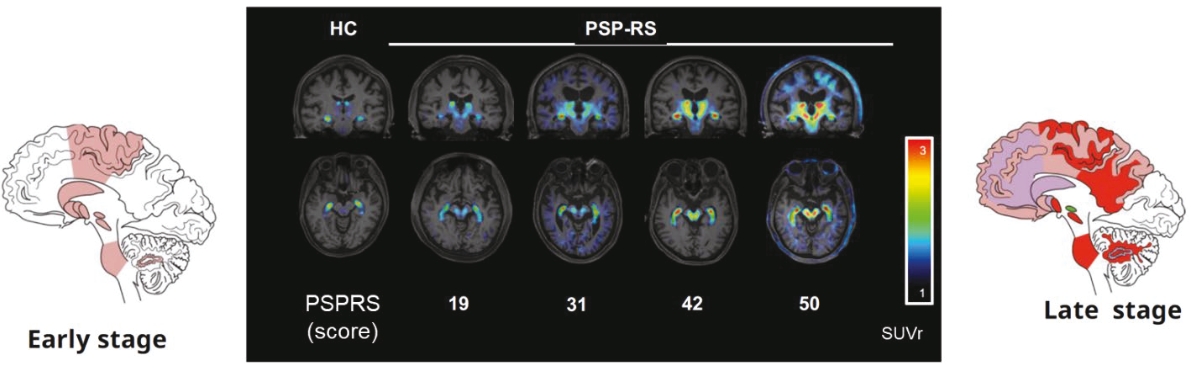
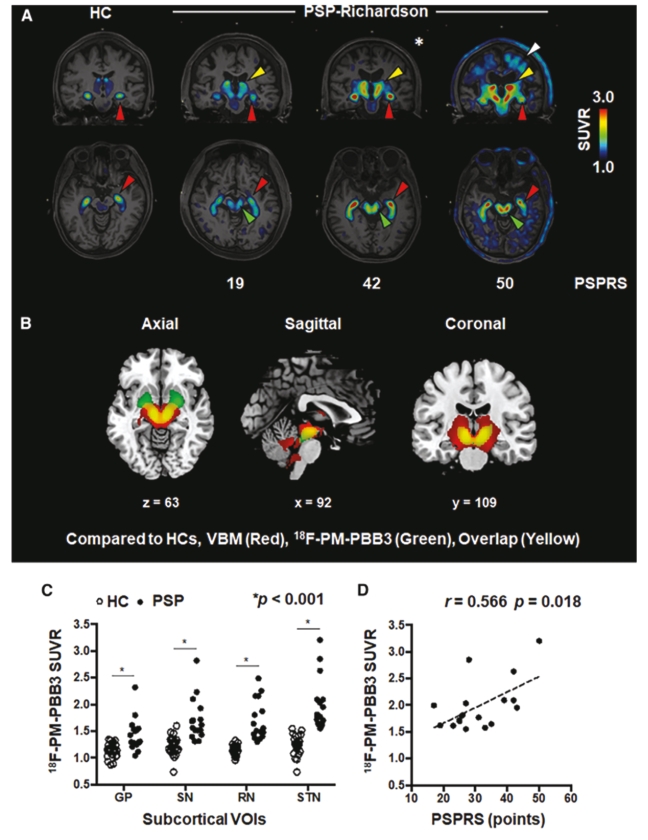
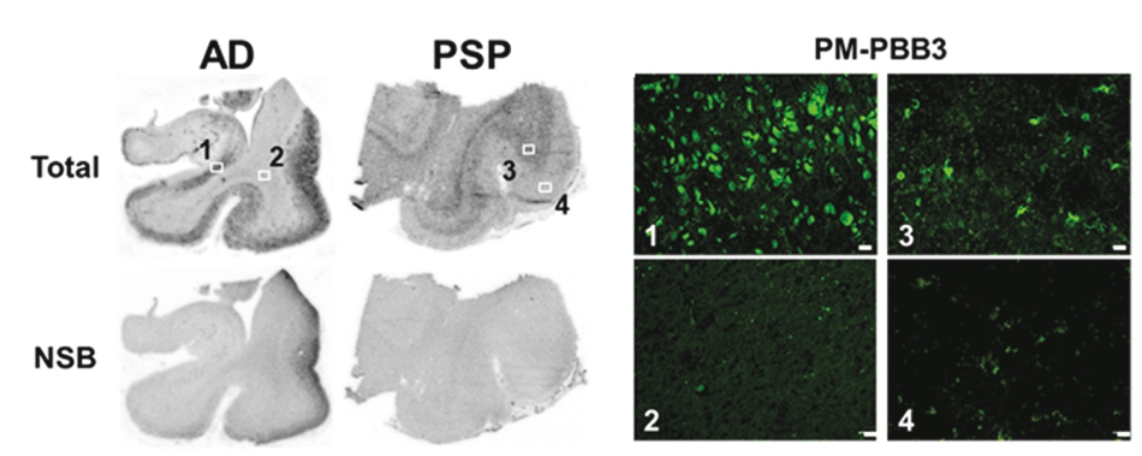
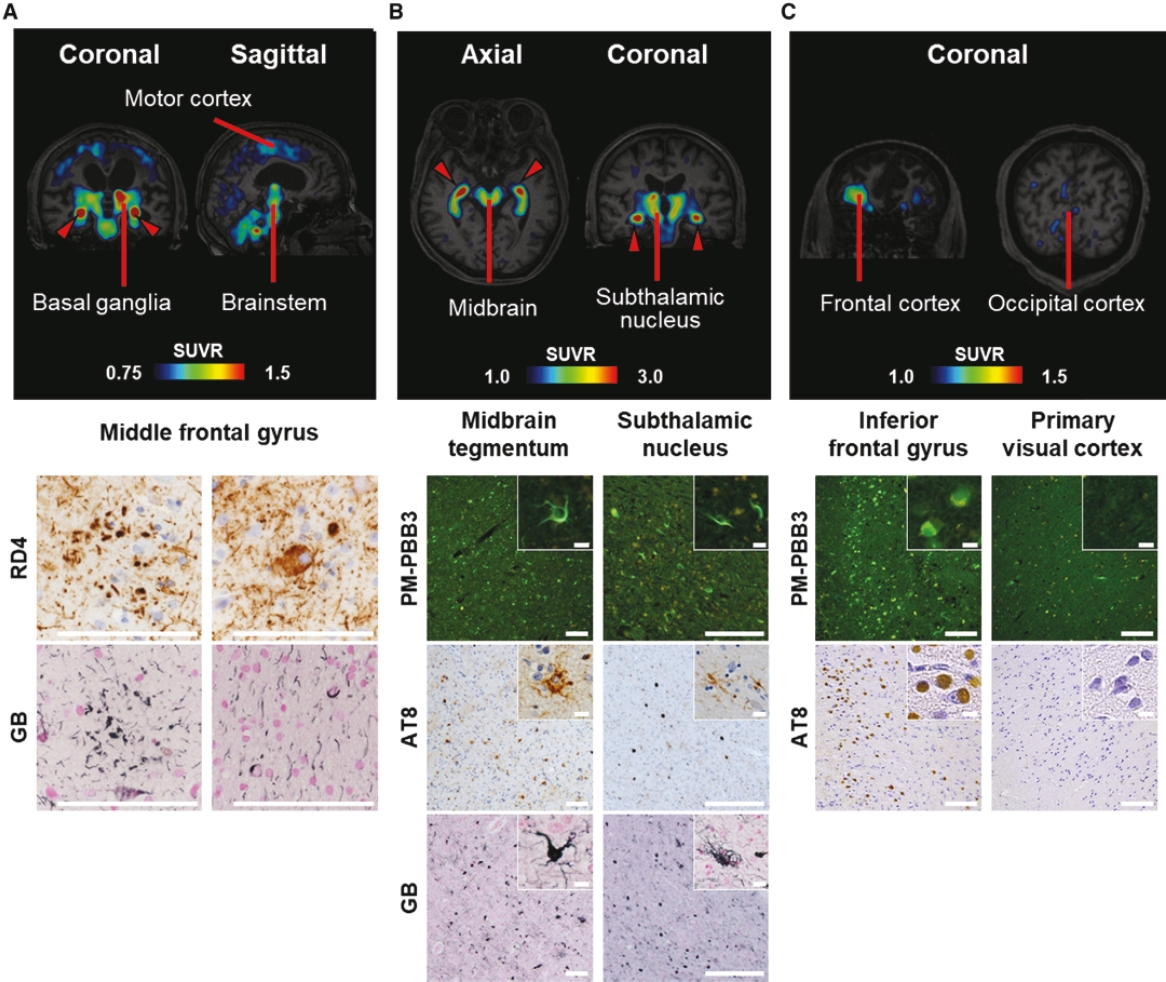
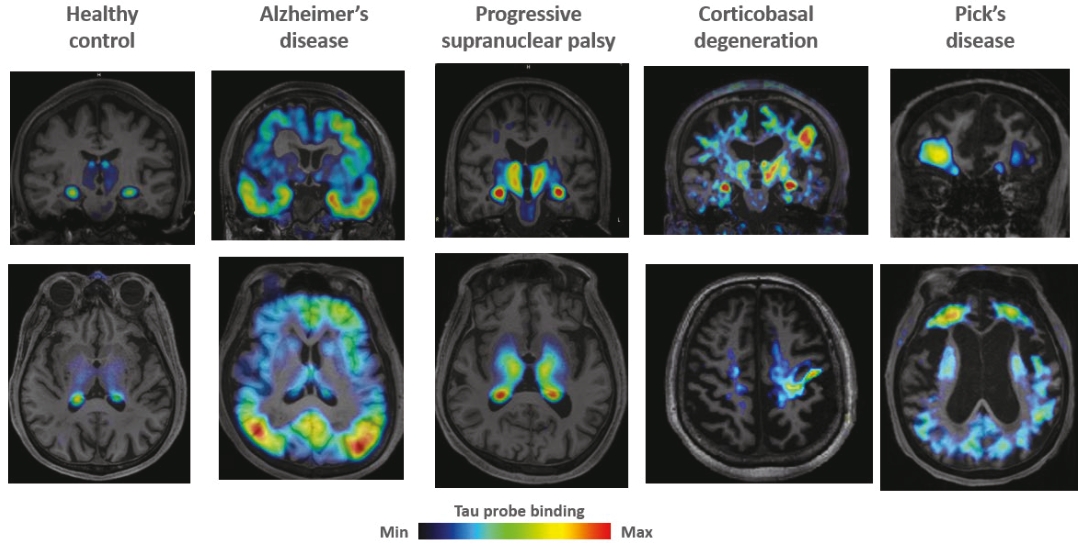
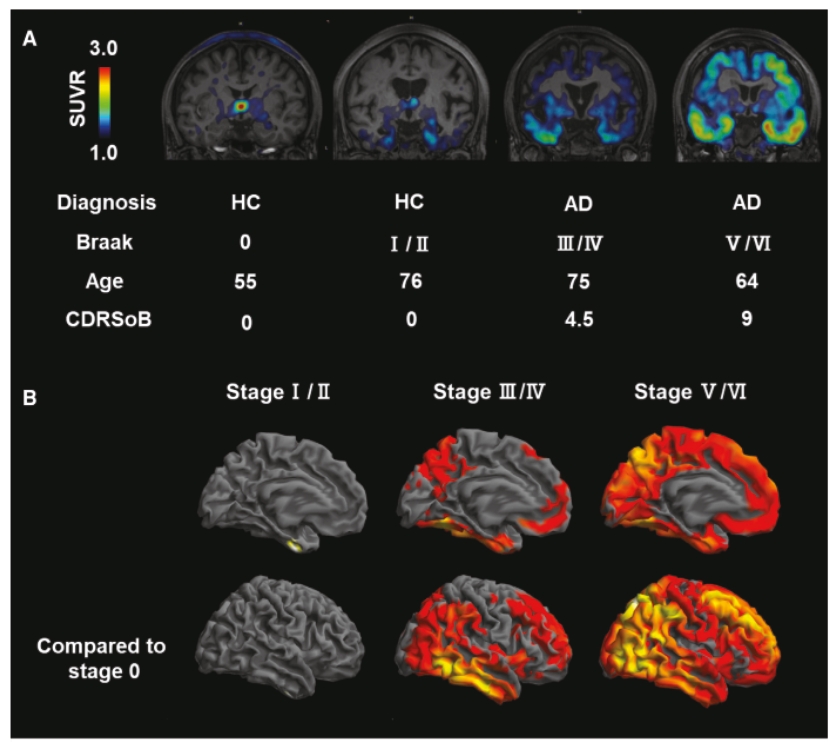
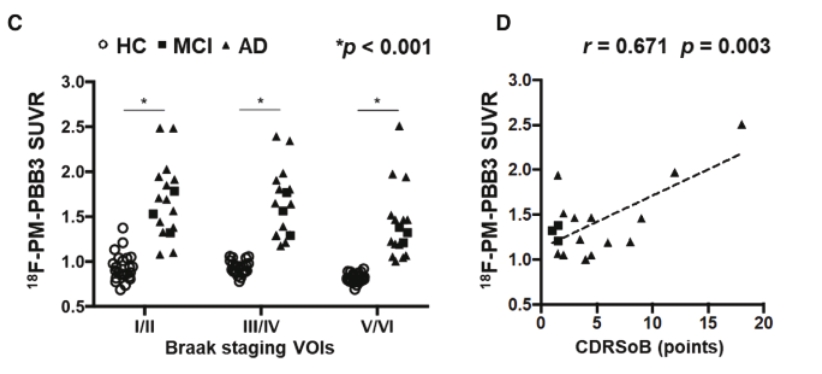
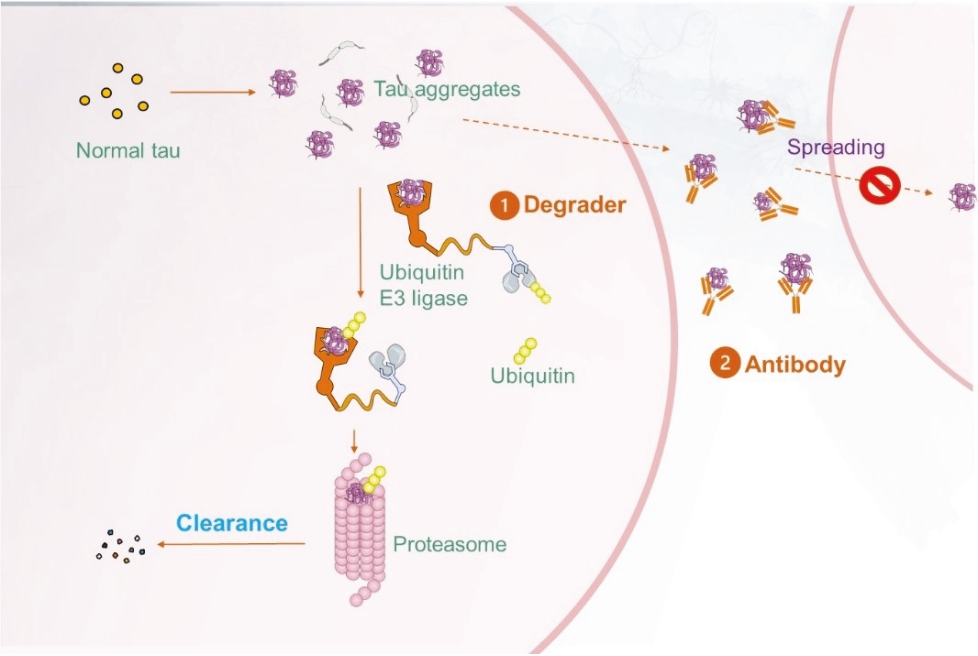
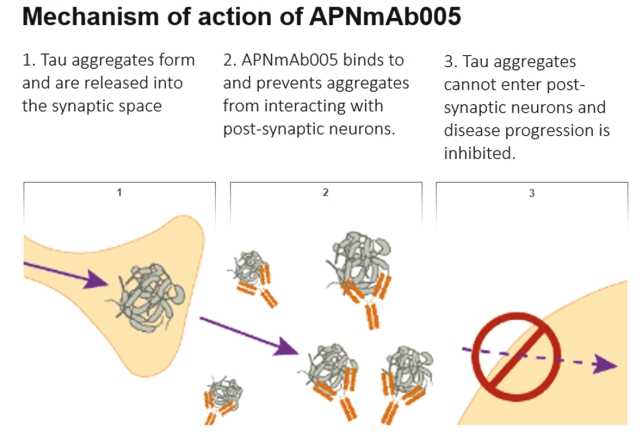
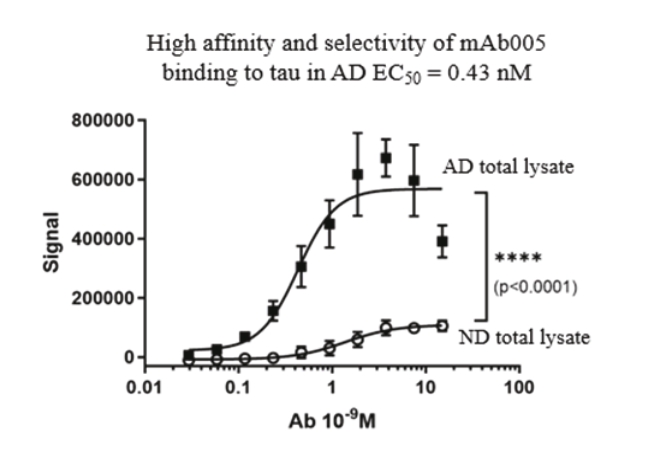
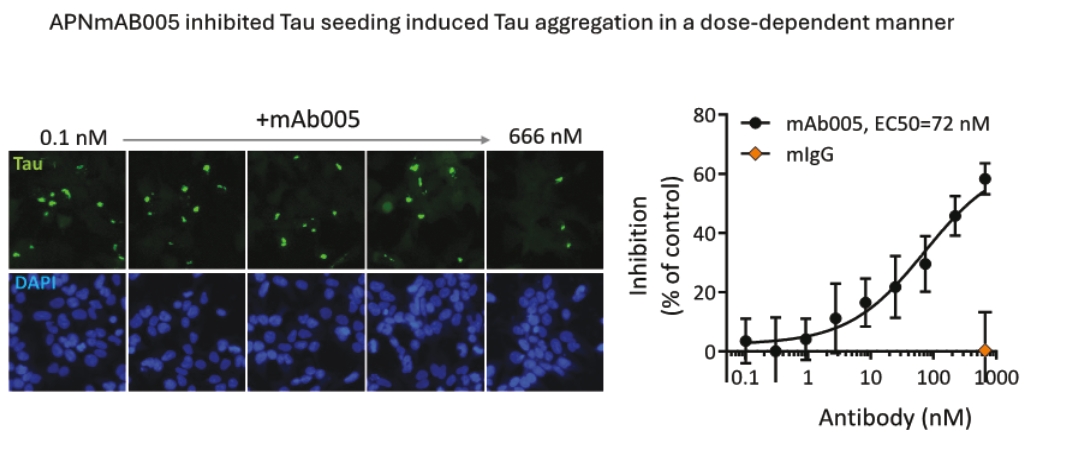
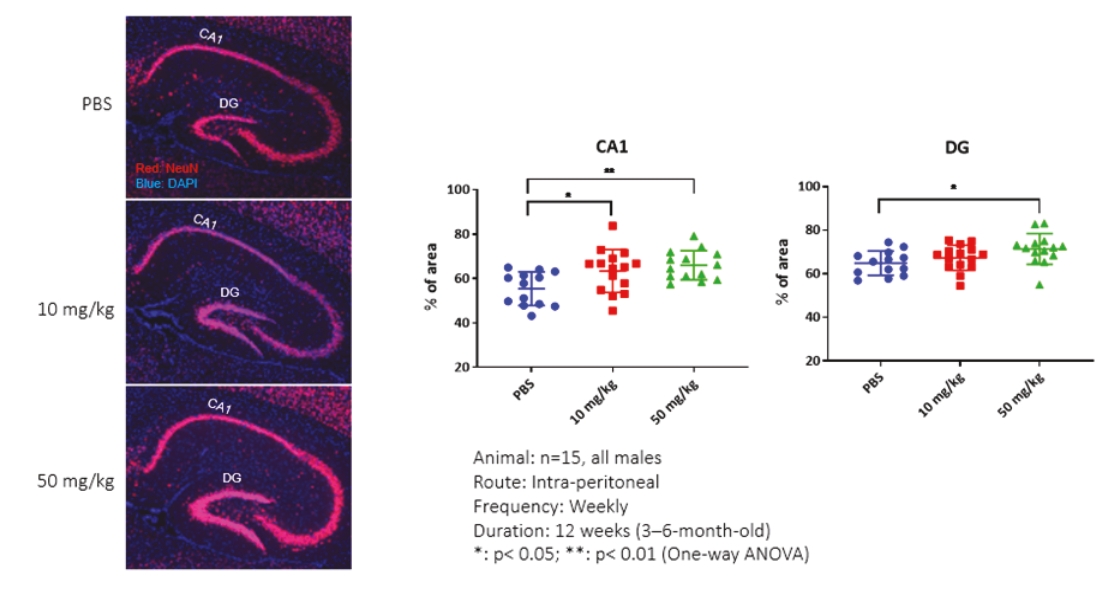
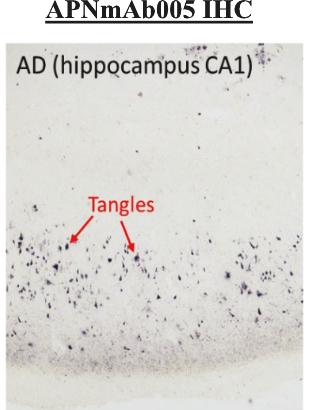
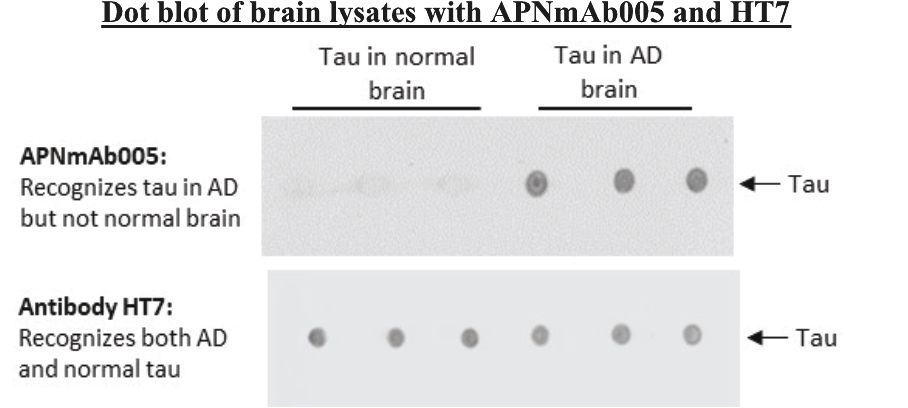
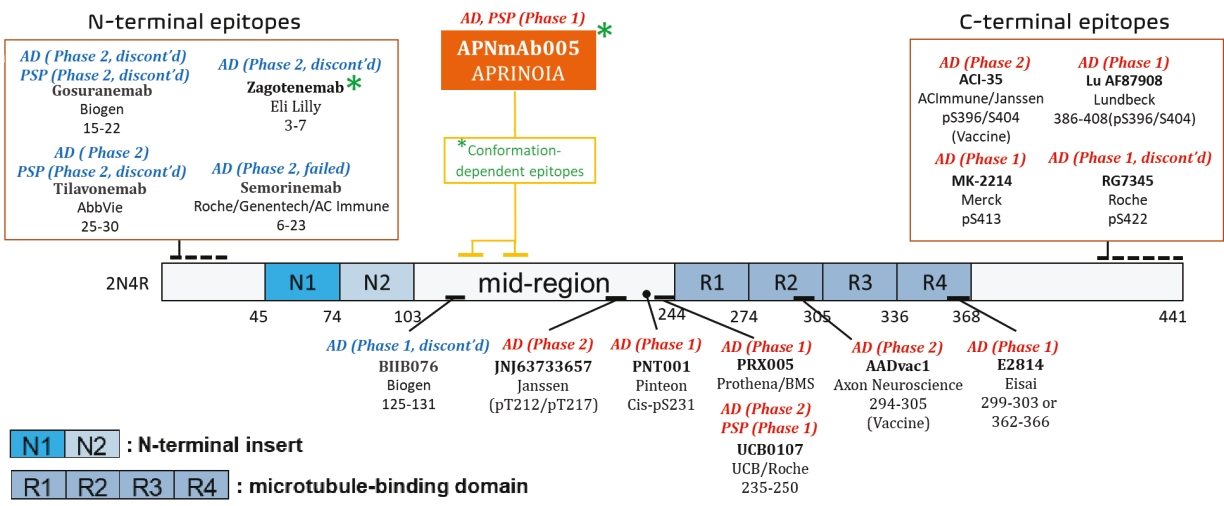
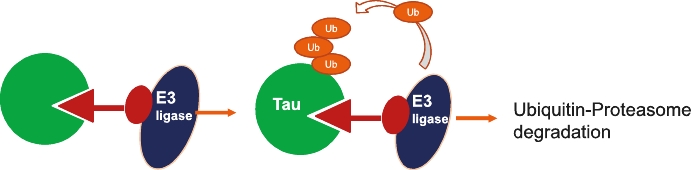
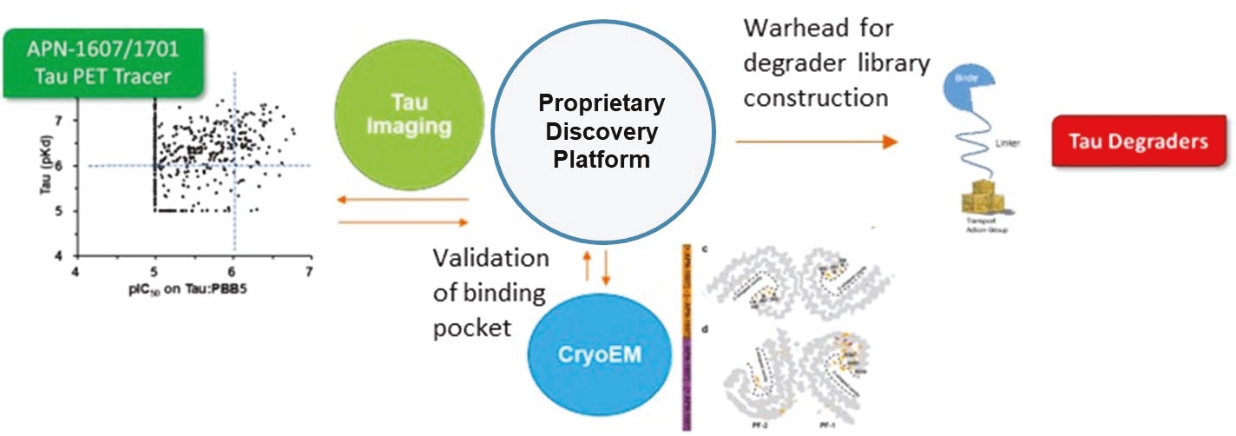
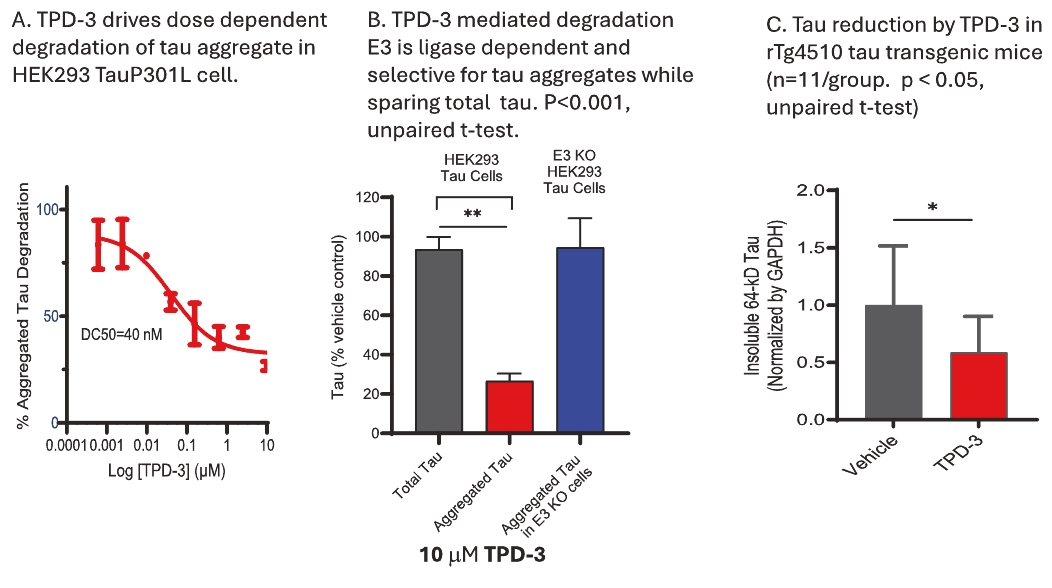
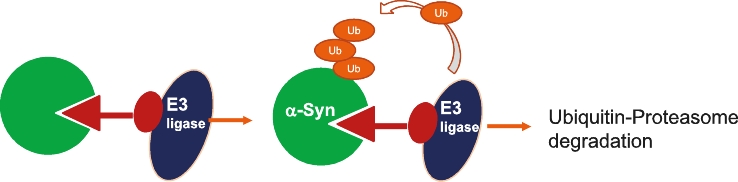
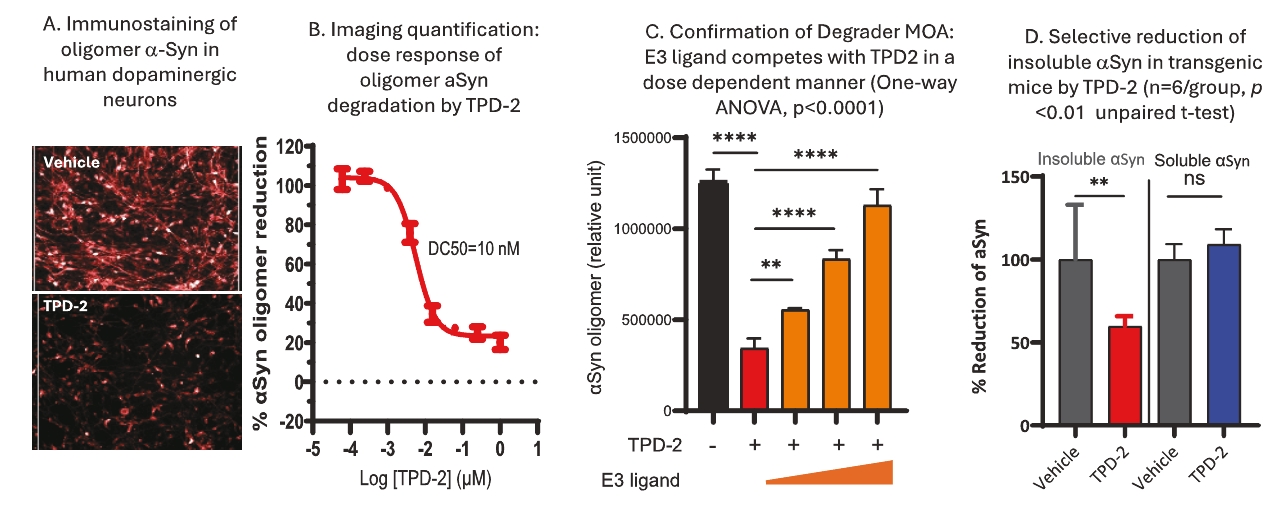
On June 8, 2021, we issued 3,371,805 Series C Preferred Shares to an existing shareholder for an aggregate consideration of approximately $18.1 million.
On October 14, 2021, we issued a total of 1,582,279 Series C Preferred Shares to new investors for an aggregate consideration of approximately $10.0 million.
On April 13, 2022, we issued a total of 1,424,052 Series C Preferred Shares to new investors for an aggregate consideration of approximately $9.0 million.
Options
During the past three years, we granted to certain directors, officers, employees and consultants on various dates options to purchase an aggregate of 2,875,258 ordinary shares for their past and future services to us.
Convertible Promissory Notes
On December 20 and 21, 2022, we issued convertible promissory notes to investors and one of our officers for an aggregate consideration of $1,450,000. Each note bears a simple interest of 5% per annum and is due one year after the date of note issuance.
On January 12, 2023, we issued convertible promissory notes to investors for an aggregate consideration of $10,500,000. Each note bears a simple interest of 5% per annum and is due one year after the date of note issuance. One of the notes with an aggregate consideration of $7,500,000 has optional conversion mechanism upon certain specific events.
On February 7, 2023, we issued a convertible promissory note to one of our directors for consideration of $250,000, which bears a simple interest rate of 5% per annum and is due one year after the date of note issuance.
On May 23, 2023, we issued convertible promissory notes to a new investor for an aggregate consideration of $4,357,900. Each note bears a simple interest of 5% per annum and is due one year after the date of note issuance.
On June 30, 2023, we issued a convertible promissory note to a new investor for consideration of $100,000, which bears a simple interest rate of 5% per annum accruing from February 17, 2023 and is due one year thereafter.
On November 10, 2023, we issued a convertible promissory note to a new investor for consideration of $100,000, which bears a simple interest rate of 5% per annum and is due one year after the date of note issuance.
On November 20, 2023, we issued a convertible promissory note to a new investor for consideration of $400,000, which bears a simple interest rate of 5% per annum and is due one year after the date of note issuance.
On December 12 and 18, 2023, we renewed two convertible promissory notes, with an aggregate principal amount of $1,400,000, with its respective investor. Each renewed note bears a simple interest of 5% from the issuance date of the original note and an increased simple interest of 8% per annum from the date of renewal, and is due one year after the renewal date of such note.
On December 21, 2023, a convertible promissory note that was issued to one of our officers for consideration of $50,000 matured. We paid all principal and interest due per the terms of the promissory note in January 2024.
On January 9, 2024, we issued convertible promissory notes to two new investors for an aggregate consideration of $4,000,000. Each note bears a simple interest rate of 8% per annum and is due one year after the date of note issuance. Both notes have optional conversion mechanism upon certain specific events.
On January 12, 2024, we renewed a convertible promissory note with a principal amount of $3,000,000. The renewed promissory note bears a simple interest of 5% per annum from the issuance date of the original note and an increased simple interest of 8% per annum from the date of renewal and is due one year after the renewal date.
On January 12, 2024, a convertible note with principal amount of $7,500,000 matured and we executed an agreement with the investor to extend the maturity date to February 15, 2024 for the purpose of renegotiating the terms of the promissory note. We agreed to increase the interest rate to the equivalent of 12.5% per annum during the period of extension and all other terms of the promissory note remain unchanged. In February 2024, we entered into an Amended and Restated Promissory Note Agreement and Security Agreement with R. Investments, LLC, extending the maturity date of the original promissory note to January 31, 2025, at 12% interest, and securing the promissory note with collateral comprising revenue derived from our licensing out of certain of our intellectual property.

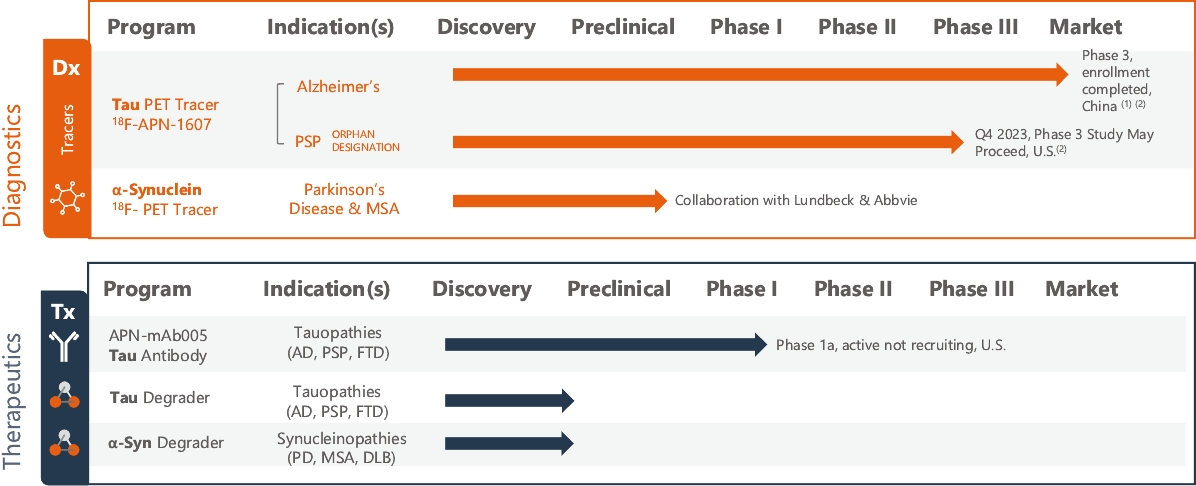
 as our wholly-owned subsidiary in Japan, and APRINOIA Therapeutics, LLC as our wholly-owned subsidiary in the United States. In December 2023, we incorporated APRINOIA Therapeutics Limited as our wholly-owned subsidiary in Ireland. Our principal executive offices are located at 245 Main Street, 2nd Floor, Cambridge, MA 02142. Our telephone number at this address is 617-225-4415. Our registered office is located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Island. Investors should submit any inquiries to the address and telephone number of our principal executive offices set forth above.
as our wholly-owned subsidiary in Japan, and APRINOIA Therapeutics, LLC as our wholly-owned subsidiary in the United States. In December 2023, we incorporated APRINOIA Therapeutics Limited as our wholly-owned subsidiary in Ireland. Our principal executive offices are located at 245 Main Street, 2nd Floor, Cambridge, MA 02142. Our telephone number at this address is 617-225-4415. Our registered office is located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Island. Investors should submit any inquiries to the address and telephone number of our principal executive offices set forth above.