DESCRIPTION OF SHARE CAPITAL
We are a Cayman Islands exempted company incorporated with limited liability and our affairs are governed by our memorandum and articles of association, as amended from time to time, the Companies Act (As Revised) of the Cayman Islands, which we refer to as the Companies Act below and the common law of the Cayman Islands.
As of the date of this prospectus, our authorized share capital is $50,000,000 divided into four classes of shares as follows: (i) 443,026,664 Ordinary Shares, (ii) 15,000,000 Series B Preferred Shares, (iii) 11,973,336 Pre-Series C Preferred Shares, and (iv) 30,000,000 Series C Preferred Shares.
As of the date of this prospectus, we had (i) 40,492,206 Ordinary Shares, (ii) 13,883,000 Series B Ordinary Shares, (iii) 11,973,336 Pre-Series C Shares, and (iv) 27,766,265 Series C Preferred Shares, in each case issued and outstanding. All of our shares issued and outstanding prior to the completion of the offering will be fully paid, and all of our shares to be issued in the offering will be issued as fully paid.
Immediately prior to the completion of this offering, our authorized share capital will be $ divided into shares, comprising of (i) ordinary shares of a par value of $ each, and (ii) undesignated shares of a par value of $ each of such class or classes (however designated) as our board of directors may determine in accordance with our [post-offering amended and restated memorandum and articles of association]. Immediately prior to the completion of this offering, all of our issued and outstanding preferred shares will be converted into ordinary shares on a [one-for-one] basis. Following such conversion, we will have ordinary shares issued and outstanding prior to the completion of this offering. All of our shares issued and outstanding prior to the completion of the offering will be fully paid, and all of our shares to be issued in this offering will be issued as fully paid.
Upon the completion of this offering, our board of directors may, without further action by our shareholders, fix the rights, preferences, privileges, and restrictions of up to an aggregate of undesignated shares, including preferred shares, in one or more classes or series and authorize their issuance. These rights, preferences, and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms, and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of our ordinary shares. The issuance of our other shares, including potentially preferred shares, could adversely affect the voting power of holders of ordinary shares and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of other shares, including preferred shares, could have the effect of delaying, deferring, or preventing a change of control or other corporate action. Upon the completion of this offering, no preferred shares will be outstanding, and we have no present plan to issue any preferred shares.
Our [Post-Offering Amended and Restated Memorandum and Articles of Association]
[Our shareholders intend to adopt an amended and restated memorandum and articles of association, which will become effective and replace our current amended and restated memorandum and articles of association in its entirety immediately prior to the completion of this offering. The following are summaries of material provisions of the sixth amended and restated memorandum and articles of association, or our [post-offering amended and restated memorandum and articles of association], that we expect will become effective immediately prior to completion of this offering, and of the Companies Act, insofar as they relate to the material terms of our ordinary shares.
Objects of Our Company. Under our [post-offering amended and restated memorandum and articles of association], the objects of our company are unrestricted and we have the full power and authority to carry out any object not prohibited by the law of the Cayman Islands.
Ordinary Shares. Our ordinary shares are issued in registered form and are issued when registered in our register of members. We may not issue shares to bearer. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their shares.
Dividends. The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors. In addition, our shareholders may declare dividends by ordinary resolution, but no dividend may exceed the amount recommended by our directors. Our [post-offering amended and restated memorandum and articles of association] provide that the directors may, before recommending or declaring any dividend, set aside out of the funds legally available for distribution such sums as they think proper as a reserve or reserves which shall, in the absolute discretion of the directors, be applicable for meeting contingencies or for equalizing dividends or for any other purpose to which those funds may be properly applied. Under the laws of the Cayman Islands, our company may pay


 as our wholly-owned subsidiary in Japan, and APRINOIA Therapeutics, LLC as our wholly-owned subsidiary in the United States. Our principal executive offices are located at 245 Main Street, 2nd Floor, Cambridge, MA 02142. Our telephone number at this address is 617-225-4415. Our registered office is located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Island. Investors should submit any inquiries to the address and telephone number of our principal executive offices set forth above.
as our wholly-owned subsidiary in Japan, and APRINOIA Therapeutics, LLC as our wholly-owned subsidiary in the United States. Our principal executive offices are located at 245 Main Street, 2nd Floor, Cambridge, MA 02142. Our telephone number at this address is 617-225-4415. Our registered office is located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Island. Investors should submit any inquiries to the address and telephone number of our principal executive offices set forth above. 


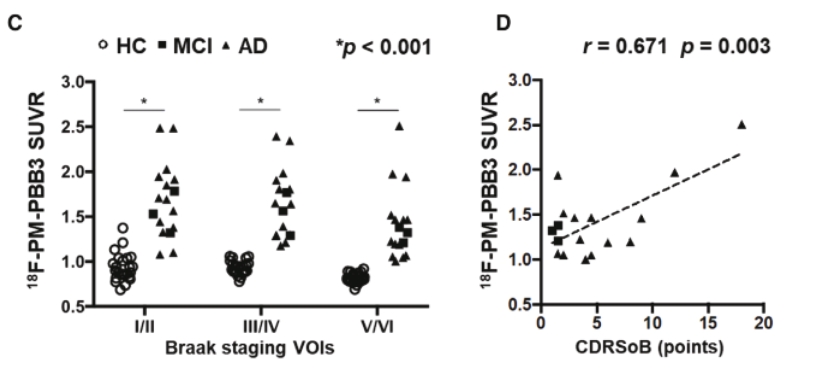

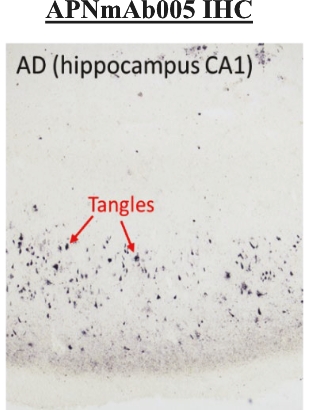





 in Japan (“APRINOIA Japan”), APRINOIA Therapeutics Limited in Hong Kong (“APRINOIA HK”) and APRINOIA Therapeutics, LLC in the United States (“APRINOIA USA”). APRINOIA HK has a direct wholly-owned subsidiary, Suzhou APRINOIA Therapeutics Co, Ltd., in Suzhou (“APRINOIA Suzhou”). The Company had a subsidiary in Taiwan (APRINOIA Therapeutics Inc. (Taiwan) or “APRINOIA Taiwan”), which was inactive in late 2021 and dissolved on May 20, 2022.
in Japan (“APRINOIA Japan”), APRINOIA Therapeutics Limited in Hong Kong (“APRINOIA HK”) and APRINOIA Therapeutics, LLC in the United States (“APRINOIA USA”). APRINOIA HK has a direct wholly-owned subsidiary, Suzhou APRINOIA Therapeutics Co, Ltd., in Suzhou (“APRINOIA Suzhou”). The Company had a subsidiary in Taiwan (APRINOIA Therapeutics Inc. (Taiwan) or “APRINOIA Taiwan”), which was inactive in late 2021 and dissolved on May 20, 2022.