UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other Jurisdiction of Incorporation) |
(Commission File No.) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices, including Zip Code)
(Registrant’s telephone number, including area code)
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 8.01 | Other Events. |
On January 25, 2024, Harpoon Therapeutics, Inc., a Delaware corporation (“Harpoon”), presented a clinical update at the American Society of Clinical Oncology (“ASCO”) Genitourinary Cancers Symposium. A copy of the slides, which has been published on Harpoon’s website, is filed as Exhibit 99.1 to this current report on Form 8-K and is incorporated by reference herein.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| 99.1 | Slides from Harpoon Therapeutics, Inc.’s ASCO Genitourinary Cancers Symposium Presentation, dated January 25, 2024. | |
| 104 | Cover Page Interactive Data file (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Harpoon Therapeutics, Inc. | ||
| By: | /s/ Julie Eastland | |
| Name: Julie Eastland | ||
| Title: President and Chief Executive Officer | ||
Dated: January 25, 2024

Interim Results from a Phase 1/2 Study of HPN328, a Tri-Specific, Half-Life (T1/2) Extended DLL3-targeting T-Cell Engager in Patients (pts) with Neuroendocrine Prostate Cancer (NEPC) and other Neuroendocrine Neoplasms (NEN) Himisha Beltran1, Afshin Dowlati2, Prantesh Jain3, Melissa Lynne Johnson4, Rachel E. Sanborn5, Jonathan Robert Thompson6, Hirva Mamdani7, Erin L. Schenk8, Rahul Raj Aggarwal9, Kamya Sankar10, Ann W. Gramza11, Banmeet S Anand11, Noura J. Choudhury12 1Dana-Farber Cancer Institute, Boston, MA; 2University Hospital Siedman Cancer Center, Case Western Reserve University, Cleveland, OH; 3Roswell Park Comprehensive Cancer Center, Buffalo, NY; 4Sarah Cannon Research Institute, Nashville, TN; 5Earle A Chiles Research Institute, Portland, OR; 6Froedtert and the Medical College of Wisconsin Workforce Health, Milwaukee, WI; 7Barbara Ann Karmanos Cancer Institute, Detroit, MI; 8University of Colorado - Anschutz Medical Campus, Aurora, CO; 9University of California, San Francisco, San Francisco, CA; 10Cedars-Sinai Medical Center, Los Angeles, CA; 11Harpoon Therapeutics, Inc., South San Francisco, CA; 12Memorial Sloan Kettering Cancer Center, New York, NY Dr. Himisha Beltran Exhibit 99.1
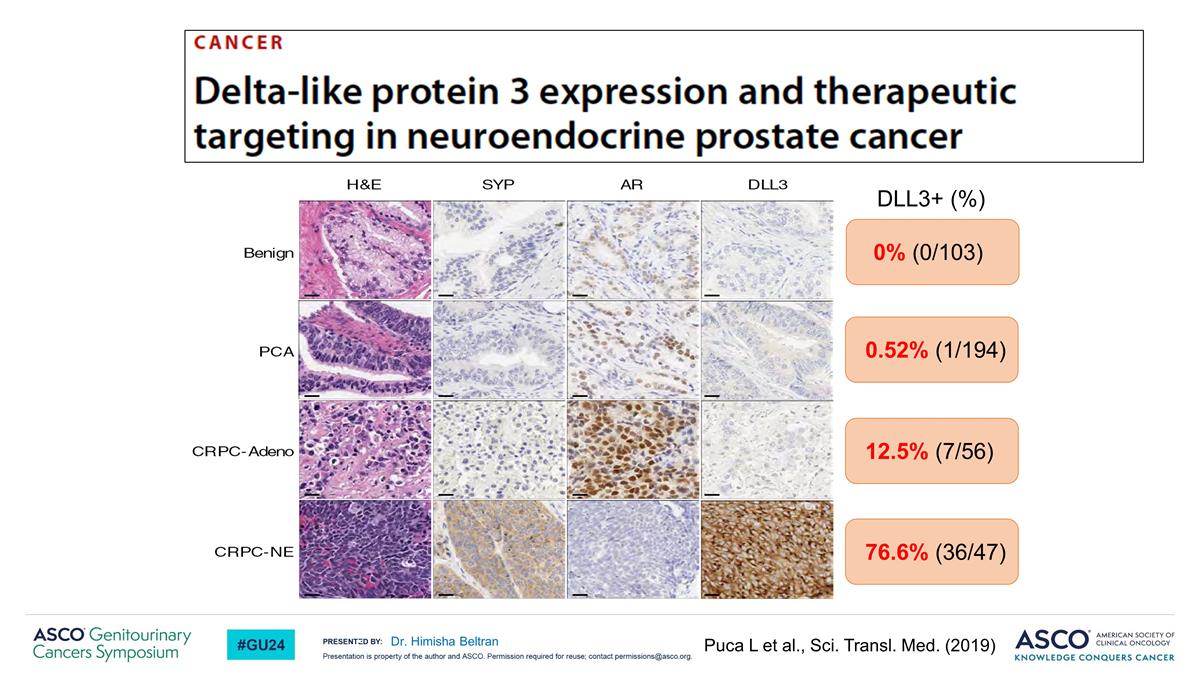
76.6% (36/47) 12.5% (7/56) 0.52% (1/194) 0% (0/103) DLL3+ (%) Puca L et al., Sci. Transl. Med. (2019) Dr. Himisha Beltran
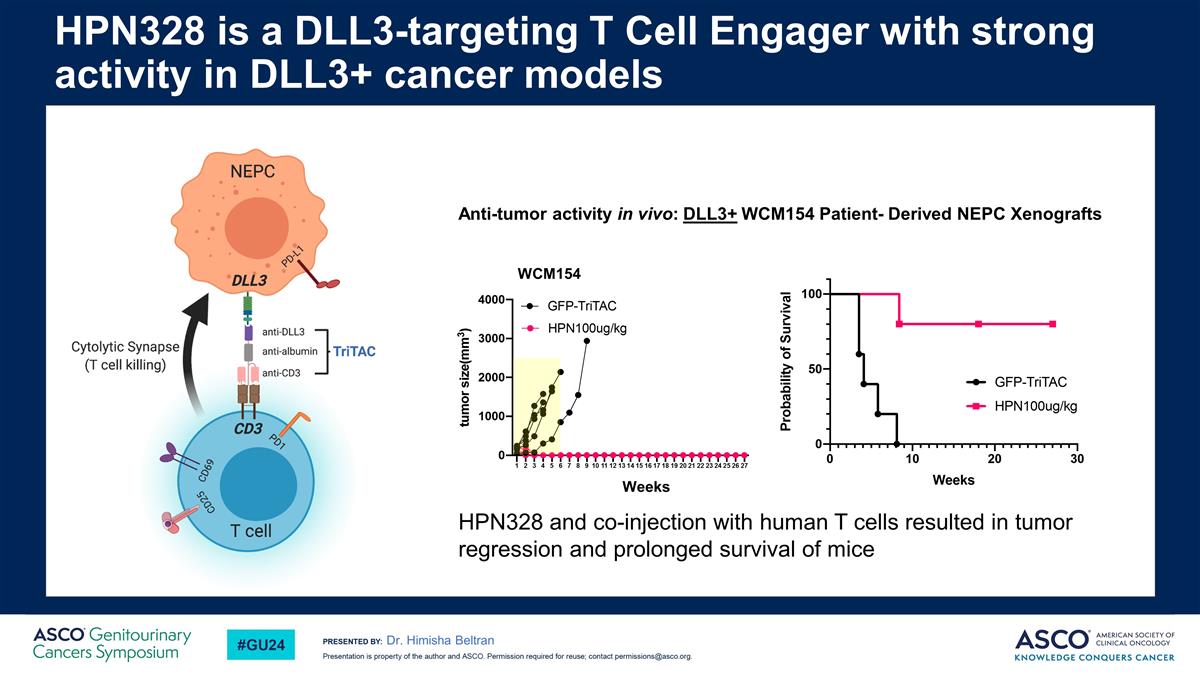
Dr. Himisha Beltran HPN328 is a DLL3-targeting T Cell Engager with strong activity in DLL3+ cancer models WCM154 Weeks Anti-tumor activity in vivo: DLL3+ WCM154 Patient- Derived NEPC Xenografts HPN328 and co-injection with human T cells resulted in tumor regression and prolonged survival of mice
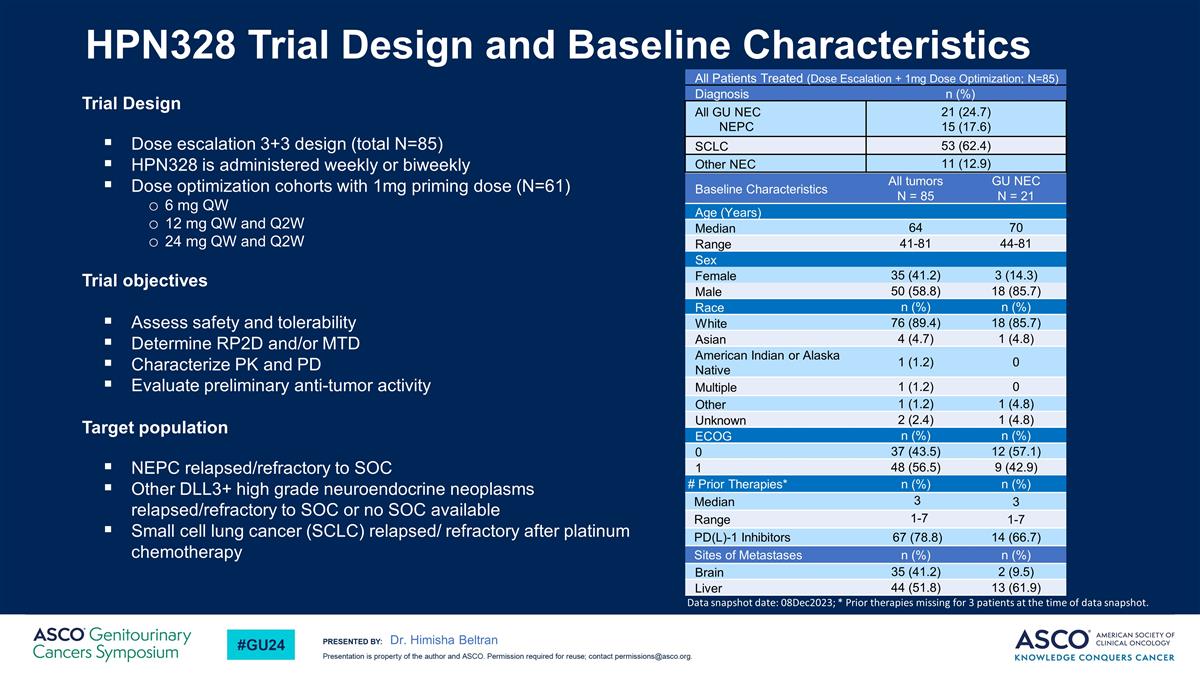
HPN328 Trial Design and Baseline Characteristics Trial Design Dose escalation 3+3 design (total N=85) HPN328 is administered weekly or biweekly Dose optimization cohorts with 1mg priming dose (N=61) 6 mg QW 12 mg QW and Q2W 24 mg QW and Q2W Trial objectives Assess safety and tolerability Determine RP2D and/or MTD Characterize PK and PD Evaluate preliminary anti-tumor activity Target population NEPC relapsed/refractory to SOC Other DLL3+ high grade neuroendocrine neoplasms relapsed/refractory to SOC or no SOC available Small cell lung cancer (SCLC) relapsed/ refractory after platinum chemotherapy Baseline Characteristics All tumors N = 85 GU NEC N = 21 Age (Years) Median 64 70 Range 41-81 44-81 Sex Female 35 (41.2) 3 (14.3) Male 50 (58.8) 18 (85.7) Race n (%) n (%) White 76 (89.4) 18 (85.7) Asian 4 (4.7) 1 (4.8) American Indian or Alaska Native 1 (1.2) 0 Multiple 1 (1.2) 0 Other 1 (1.2) 1 (4.8) Unknown 2 (2.4) 1 (4.8) ECOG n (%) n (%) 0 37 (43.5) 12 (57.1) 1 48 (56.5) 9 (42.9) # Prior Therapies* n (%) n (%) Median 3 3 Range 1-7 1-7 PD(L)-1 Inhibitors 67 (78.8) 14 (66.7) Sites of Metastases n (%) n (%) Brain 35 (41.2) 2 (9.5) Liver 44 (51.8) 13 (61.9) All Patients Treated (Dose Escalation + 1mg Dose Optimization; N=85) Diagnosis n (%) All GU NEC NEPC 21 (24.7) 15 (17.6) SCLC 53 (62.4) Other NEC 11 (12.9) Data snapshot date: 08Dec2023; * Prior therapies missing for 3 patients at the time of data snapshot. Dr. Himisha Beltran
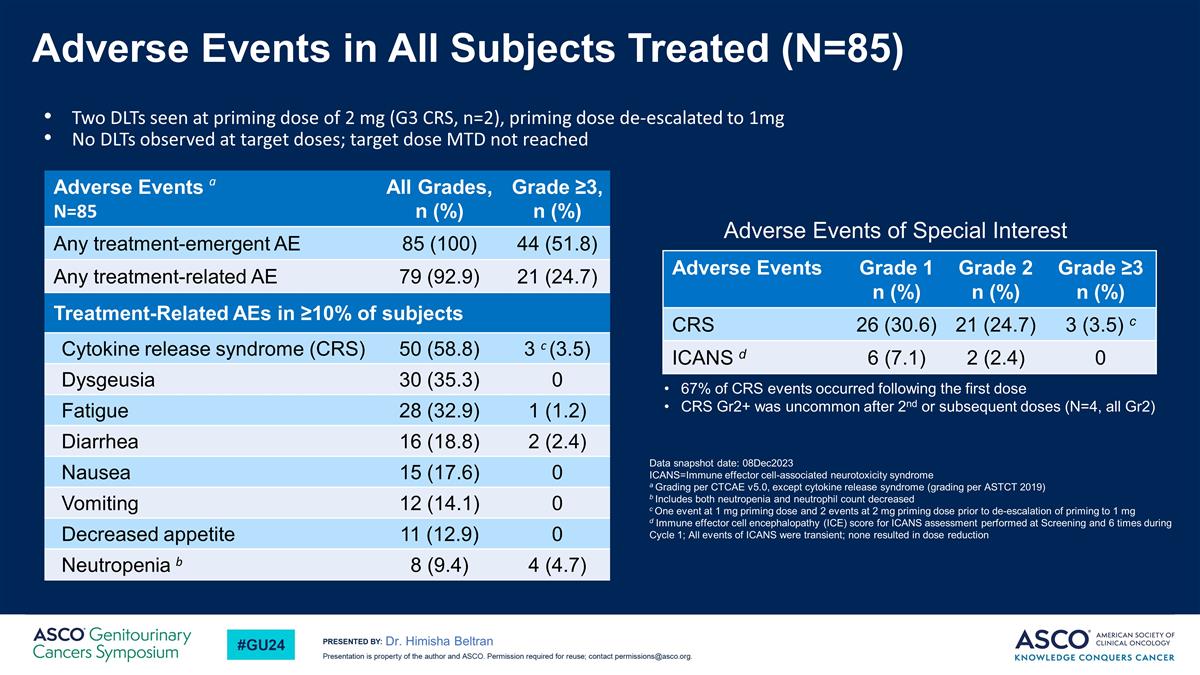
Adverse Events in All Subjects Treated (N=85) Dr. Himisha Beltran Two DLTs seen at priming dose of 2 mg (G3 CRS, n=2), priming dose de-escalated to 1mg No DLTs observed at target doses; target dose MTD not reached Data snapshot date: 08Dec2023 ICANS=Immune effector cell-associated neurotoxicity syndrome a Grading per CTCAE v5.0, except cytokine release syndrome (grading per ASTCT 2019) b Includes both neutropenia and neutrophil count decreased c One event at 1 mg priming dose and 2 events at 2 mg priming dose prior to de-escalation of priming to 1 mg d Immune effector cell encephalopathy (ICE) score for ICANS assessment performed at Screening and 6 times during Cycle 1; All events of ICANS were transient; none resulted in dose reduction Adverse Events Grade 1 n (%) Grade 2 n (%) Grade ≥3 n (%) CRS 26 (30.6) 21 (24.7) 3 (3.5) c ICANS d 6 (7.1) 2 (2.4) 0 Adverse Events a N=85 All Grades, n (%) Grade ≥3, n (%) Any treatment-emergent AE 85 (100) 44 (51.8) Any treatment-related AE 79 (92.9) 21 (24.7) Treatment-Related AEs in ≥10% of subjects Cytokine release syndrome (CRS) 50 (58.8) 3 c (3.5) Dysgeusia 30 (35.3) 0 Fatigue 28 (32.9) 1 (1.2) Diarrhea 16 (18.8) 2 (2.4) Nausea 15 (17.6) 0 Vomiting 12 (14.1) 0 Decreased appetite 11 (12.9) 0 Neutropenia b 8 (9.4) 4 (4.7) 67% of CRS events occurred following the first dose CRS Gr2+ was uncommon after 2nd or subsequent doses (N=4, all Gr2) Adverse Events of Special Interest
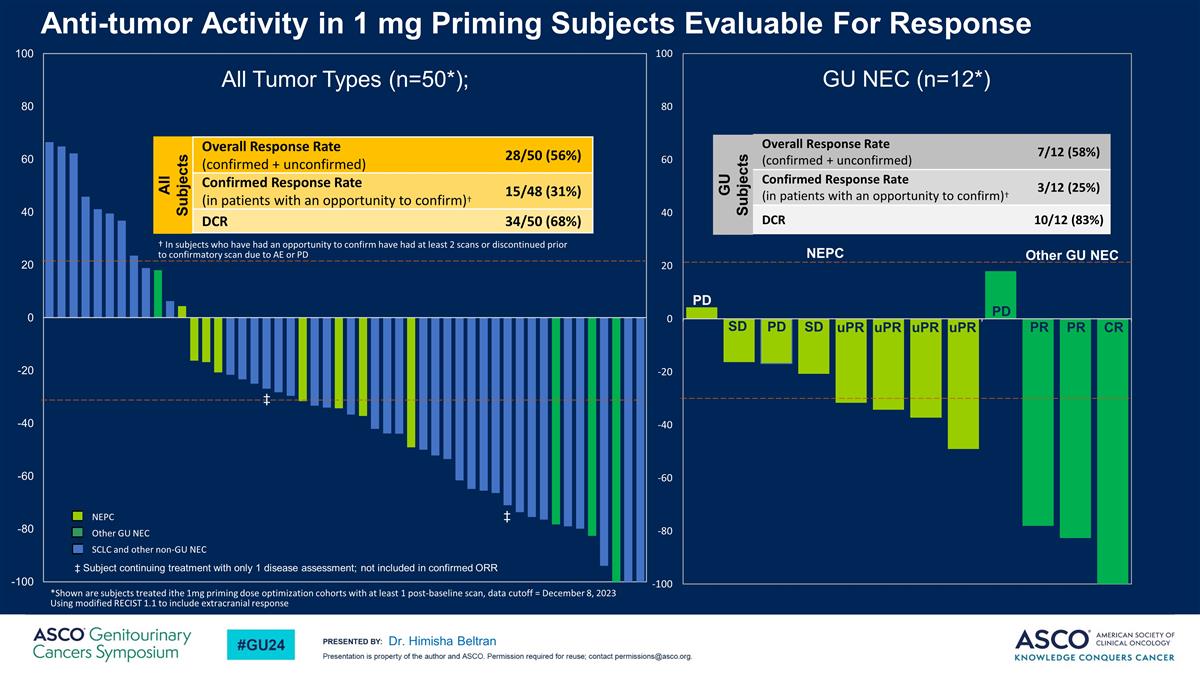
Anti-tumor Activity in 1 mg Priming Subjects Evaluable For Response Dr. Himisha Beltran All Tumor Types (n=50*); Other GU NEC *Shown are subjects treated ithe 1mg priming dose optimization cohorts with at least 1 post-baseline scan, data cutoff = December 8, 2023 Using modified RECIST 1.1 to include extracranial response GU NEC (n=12*) PD PR PR CR PD uPR uPR uPR uPR SD SD PD NEPC Overall Response Rate (confirmed + unconfirmed) 7/12 (58%) Confirmed Response Rate (in patients with an opportunity to confirm)† 3/12 (25%) DCR 10/12 (83%) GU Subjects NEPC Other GU NEC SCLC and other non-GU NEC All Subjects Overall Response Rate (confirmed + unconfirmed) 28/50 (56%) Confirmed Response Rate (in patients with an opportunity to confirm)† 15/48 (31%) DCR 34/50 (68%) † In subjects who have had an opportunity to confirm have had at least 2 scans or discontinued prior to confirmatory scan due to AE or PD ‡ Subject continuing treatment with only 1 disease assessment; not included in confirmed ORR ‡ ‡
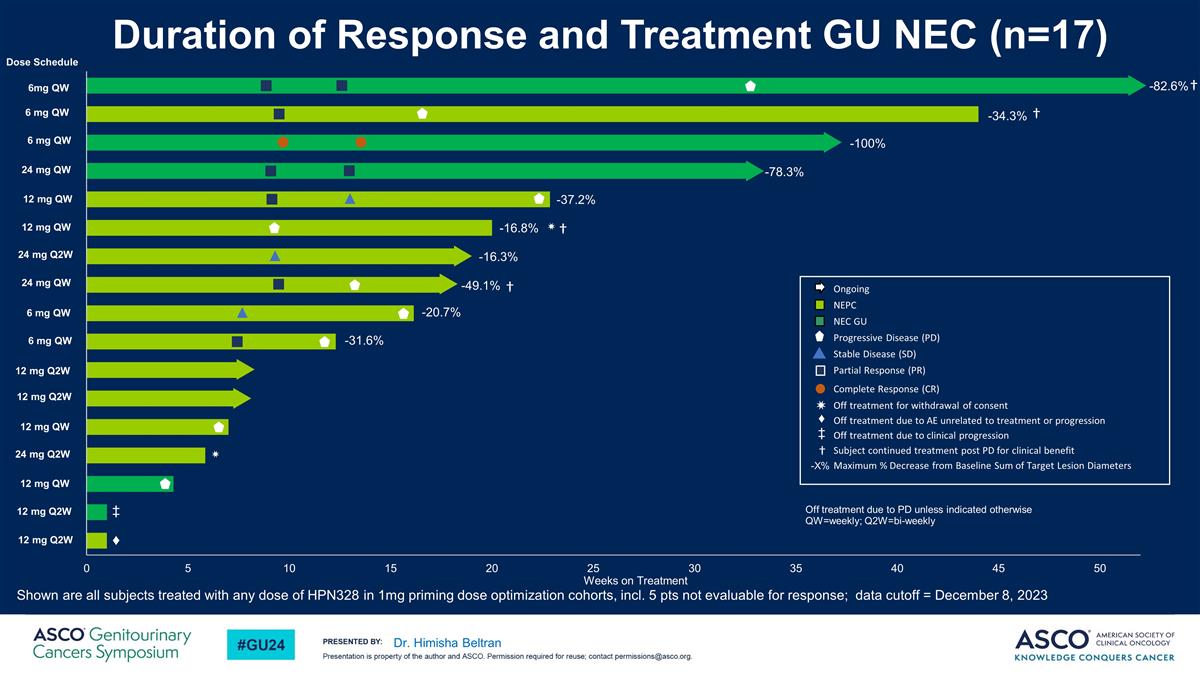
Dr. Himisha Beltran -82.6% † -34.3% -20.7% -31.6% -16.8% ✷ -78.3% -49.1% -37.2% -16.3% ✷ -100% † † Ongoing NEPC NEC GU Progressive Disease (PD) Stable Disease (SD) Partial Response (PR) Complete Response (CR) Off treatment for withdrawal of consent Off treatment due to AE unrelated to treatment or progression Off treatment due to clinical progression Subject continued treatment post PD for clinical benefit Maximum % Decrease from Baseline Sum of Target Lesion Diameters -X% Off treatment due to PD unless indicated otherwise QW=weekly; Q2W=bi-weekly 6mg QW 6 mg QW 6 mg QW 24 mg QW 12 mg QW 12 mg QW 24 mg Q2W 24 mg QW 6 mg QW 6 mg QW 12 mg Q2W 12 mg Q2W 12 mg QW 24 mg Q2W 12 mg QW 12 mg Q2W 12 mg Q2W Dose Schedule † ♦ Duration of Response and Treatment GU NEC (n=17) Shown are all subjects treated with any dose of HPN328 in 1mg priming dose optimization cohorts, incl. 5 pts not evaluable for response; data cutoff = December 8, 2023 ‡ ✷ ♦ ‡ †
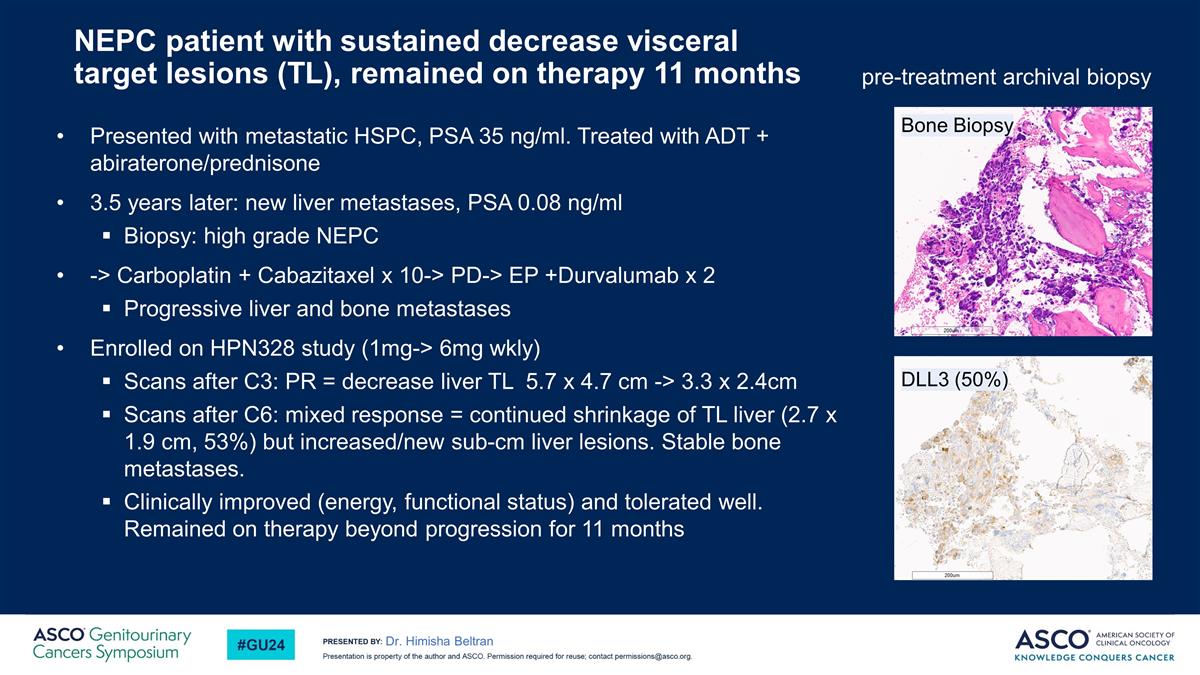
NEPC patient with sustained decrease visceral target lesions (TL), remained on therapy 11 months Presented with metastatic HSPC, PSA 35 ng/ml. Treated with ADT + abiraterone/prednisone 3.5 years later: new liver metastases, PSA 0.08 ng/ml Biopsy: high grade NEPC -> Carboplatin + Cabazitaxel x 10-> PD-> EP +Durvalumab x 2 Progressive liver and bone metastases Enrolled on HPN328 study (1mg-> 6mg wkly) Scans after C3: PR = decrease liver TL 5.7 x 4.7 cm -> 3.3 x 2.4cm Scans after C6: mixed response = continued shrinkage of TL liver (2.7 x 1.9 cm, 53%) but increased/new sub-cm liver lesions. Stable bone metastases. Clinically improved (energy, functional status) and tolerated well. Remained on therapy beyond progression for 11 months Dr. Himisha Beltran DLL3 (50%) pre-treatment archival biopsy Bone Biopsy
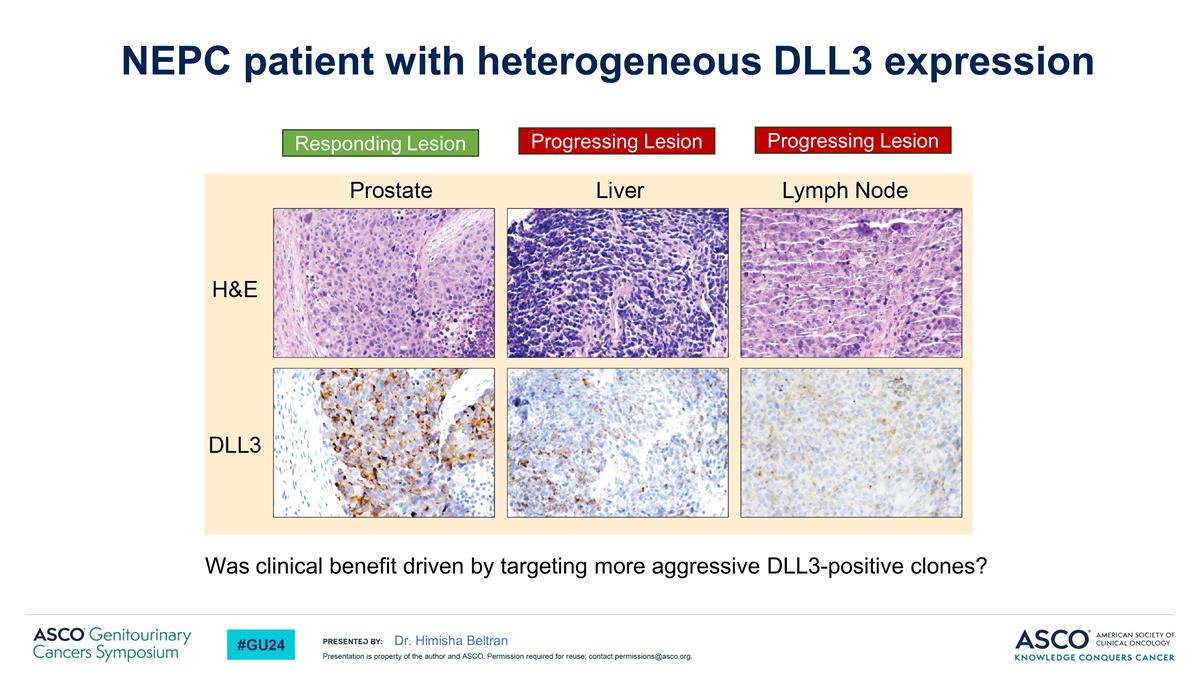
Was clinical benefit driven by targeting more aggressive DLL3-positive clones? Dr. Himisha Beltran NEPC patient with heterogeneous DLL3 expression Responding Lesion Progressing Lesion Progressing Lesion
DLL3 Expression For NEPC and SCLC, DLL3 pre-screening not required Small cell bladder cancer (N=3); DLL3 expressed in 100% tumor cells For NEPC: Sites of archival tissue: prostate, lymph node, liver, bone, pleura NEPC pathology: pure small cell carcinoma, high grade NEC, mixed adeno/small cell (with small cell component >80-90%) All 15 NEPC patients had tumors positive for DLL3 Range of DLL3 expression was 20-100% tumor cells No obvious correlation between %DLL3 positivity and response to therapy Were mixed responses due to DLL3 heterogeneity? Can benefit seen by targeting more aggressive DLL3 positive clones? Potential for combination strategies? Additional biomarker analyses and clinical correlations ongoing Dr. Himisha Beltran
Dr. Himisha Beltran Conclusions HPN328 is a novel DLL3 T cell engager that is well tolerated and demonstrates promising clinical activity in NE carcinomas, including GU NEC Adverse events were manageable, with CRS most commonly occurring with the first dose, mostly grades 1 or 2 Monotherapy dose optimization ongoing; maturing data in 12 and 24 mg cohorts will inform selection of RP2D

Acknowledgements Thank you to our patients, their families, and our clinical site staff who continue to make this trial possible. Dr. Himisha Beltran Please scan this quick response (QR) code with your camera to view this presentation and other posters and presentations on HPN328 Copies of this slide deck obtained through Quick Response (QR) Code are for personal use only and may not be reproduced without permission from ASCO® or the author of these slides