
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
|
(Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 2.02. | Results of Operations and Financial Condition. |
On January 9, 2024, Natera, Inc. (the “Company”) issued a press release announcing preliminary revenue results for its fourth quarter and fiscal year ended December 31, 2023. The Company also provided an investor presentation containing certain preliminary financial results for the same periods. A copy of the press release and a copy of the investor presentation are furnished herewith as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K and incorporated herein by reference.
The information in this Current Report on Form 8-K and the accompanying Exhibit 99.1 and Exhibit 99.2 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing, unless expressly incorporated by reference in such filing.
|
Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
|
Exhibit No.
|
Description
| |
| 99.1 | Press Release dated January 9, 2024. | |
| 99.2 | Investor Presentation. | |
| 104 | Cover Page Interactive Data File (formatted as inline XBRL). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Natera, Inc. | ||
| By: |
/s/ Michael Brophy | |
| Michael Brophy | ||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||
Dated: January 9, 2024
Exhibit 99.1

Natera Announces Preliminary Fourth Quarter and
Full Year 2023 Revenues
2023 revenues are expected to be approximately $20 million above top end of guidance
AUSTIN, Texas, Jan. 9, 2024 — Natera, Inc. (NASDAQ: NTRA), a global leader in cell-free DNA (cfDNA) testing, today announced preliminary revenue results for the fourth quarter and full year ended Dec. 31, 2023. The Company expects the following:
| ● | Total revenues of approximately $300 million in the fourth quarter of 2023, an increase of approximately 38% compared to $217 million in the fourth quarter of 2022. |
| ● | Total revenues of approximately $1.07 billion in 2023, an increase of approximately 30% compared to $820 million in 2022. |
These results are also included in a presentation that has been posted to the investor relations section of the Natera website at investor.natera.com. The presentation is being delivered today, at the 42nd Annual J.P. Morgan Healthcare Conference.
Natera plans to release its complete fourth quarter and full year 2023 financial results during its earnings call in February 2024.
About Natera
Natera™ is a global leader in cell-free DNA testing, dedicated to oncology, women’s health, and organ health. We aim to make personalized genetic testing and diagnostics part of the standard of care to protect health, and inform earlier, more targeted interventions that help lead to longer, healthier lives. Natera’s tests are validated by more than 180 peer-reviewed publications that demonstrate high accuracy. Natera operates ISO 13485-certified and CAP-accredited laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) in Austin, Texas and San Carlos, California. For more information, visit www.natera.com.
Forward-Looking Statements
This release contains forward-looking statements, including our preliminary operational and financial results for the fourth quarter and fiscal year ended December 31, 2023. The preliminary operational and financial results for the fourth quarter and fiscal year ended December 31, 2023 have not been audited by our independent registered public accounting firm and are based on management’s initial review of our operations and results for the completed fiscal year. These preliminary operational and financial results are subject to revision based upon our year-end closing procedures, final adjustments and the audit to be conducted by our independent registered public accounting firm. As a result, our actual operational and financial results may differ materially from these preliminary results. In addition, these preliminary operational and financial results are not a comprehensive statement of our operational and financial results for the fourth quarter and for fiscal 2023, and should not be viewed as a substitute for full, audited financial statements prepared in accordance with generally accepted accounting principles. Any forward-looking statements contained in this release are based upon Natera's current plans, estimates, and expectations as of the date of this release, and are not a representation that such plans, estimates, or expectations will be achieved. Subsequent events may cause these expectations to change, and Natera disclaims any obligation to update the forward-looking statements in the future.
Our forward-looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially, including: our preliminary operational and financial results for the fourth quarter and for fiscal 2023 are subject to material changes and adjustments as noted above; we face numerous uncertainties and challenges in achieving our financial projections and goals; we have incurred losses since our inception and we anticipate that we will continue to incur losses for the foreseeable future; our quarterly results may fluctuate from period to period; our estimates of market opportunity and forecasts of market growth may prove to be inaccurate. Additional risks and uncertainties that could affect our financial results are discussed in greater detail in the sections titled "Risk Factors" and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our most recent filings on Forms 10-K and 10-Q and in other filings that we make with the SEC from time to time. These documents are available at www.investor.natera.com and on the SEC’s website at www.sec.gov.
Contacts
Investor Relations: Mike Brophy, CFO, Natera, Inc., 510-826-2350, investor@natera.com
Media: Lesley Bogdanow, VP of Corporate Communications, Natera, Inc., pr@natera.com
Exhibit 99.2

Natera, Inc. Investor presentation J.P. Morgan Healthcare Conference January 9, 2024

Not for reproduction or further distribution. This presentation contains forward - looking statements under the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including statements regarding our market opportunity, our anticipated products and launch schedules, our reimbursement coverage and our product costs, our commercial and strategic partnerships and potential acquisitions, our user experience, our clinical trials and studies, our strategies, our goals and general business and market conditions, and our preliminary financial results for the fourth quarter and fiscal year ended December 31, 2023, are forward - looking statements. The preliminary financial results for the fourth quarter and fiscal year ended December 31, 2023 have not been audited by our independent registered public accounting firm and are based on management’s initial review of our operations and results for the completed fiscal year. These preliminary financial resu lts are subject to revision based upon our year - end closing procedures, final adjustments and the audit to be conducted by our independent registered public accounting firm. As a result, our actual financial results for th e fourth quarter and fiscal year ended December 31, 2023 may differ materially from these preliminary results. In addition, these preliminary financial results are not a comprehensive statement of our financial results for the fis cal year ended December 31, 2023, and should not be viewed as a substitute for full, audited financial statements prepared in accordance with generally accepted accounting principles. These forward - looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially, including : our preliminary financial results for fourth quarter and fiscal year ended December 31 , 2023 are subject to material changes and adjustments as noted above ; we face numerous uncertainties and challenges in achieving our financial projections and goals ; we may be unable to further increase the use and adoption of our products through our direct sales efforts or through our laboratory partners ; we have incurred losses since our inception and we anticipate that we will continue to incur losses for the foreseeable future ; our quarterly results may fluctuate from period to period ; our estimates of market opportunity and forecasts of market growth may prove to be inaccurate ; we may be unable to compete successfully with existing or future products or services offered by our competitors ; we may engage in acquisitions, dispositions or other strategic transactions that may not achieve our anticipated benefits and could otherwise disrupt our business, cause dilution to our stockholders or reduce our financial resources ; we may not be successful in commercializing our cloud - based distribution model ; our products may not perform as expected ; the results of our clinical studies, including our SNP - based Microdeletion and Aneuploidy Registry, or SMART, Study, may not be compelling to professional societies or payors as supporting the use of our tests, particularly for microdeletions screening, or may not be able to be replicated in later studies required for regulatory approvals or clearances ; if either of our primary CLIA - certified laboratories becomes inoperable, we will be unable to perform our tests and our business will be harmed ; we rely on a limited number of suppliers or, in some cases, single suppliers, for some of our laboratory instruments and materials and may not be able to find replacements or immediately transition to alternative suppliers ; if we are unable to successfully scale our operations, our business could suffer ; the marketing, sale, and use of Panorama and our other products could result in substantial damages arising from product liability or professional liability claims that exceed our resources ; we may be unable to expand, obtain or maintain third - party payer coverage and reimbursement for Panorama, Horizon and our other tests, and we may be required to refund reimbursements already received ; third - party payers may withdraw coverage or provide lower levels of reimbursement due to changing policies, billing complexities or other factors ; if the FDA were to begin actively regulating our tests, we could incur substantial costs and delays associated with trying to obtain premarket clearance or approval and incur costs associated with complying with post - market controls ; litigation or other proceedings, resulting from either third party claims of intellectual property infringement or third party infringement of our technology, is costly, time - consuming and could limit our ability to commercialize our products or services ; any inability to effectively protect our proprietary technology could harm our competitive position or our brand ; and we cannot guarantee that we will be able to service and comply with our outstanding debt obligations or achieve our expectations regarding the conversion of our outstanding convertible notes . We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our periodic reports on Forms 10 - K and 10 - Q and in other filings we make with the SEC from time to time . Moreover, we operate in a very competitive and rapidly changing environment . New risks emerge from time to time . It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statement . In light of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this presentation may not occur and our actual results could differ materially and adversely from those anticipated or implied . As a result, you should not place undue reliance on our forward - looking statements . Except as required by law, we undertake no obligation to update publicly any forward - looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations . We file reports, proxy statements, and other information with the SEC . Such reports, proxy statements, and other information concerning us is available at http : //www . sec . gov . Requests for copies of such documents should be directed to our Investor Relations department at Natera , Inc . , 13011 McCallen Pass, Building A Suite 100 , Austin, TX 78753 . Our telephone number is ( 650 ) 980 - 9190 . 2 Safe harbor statement
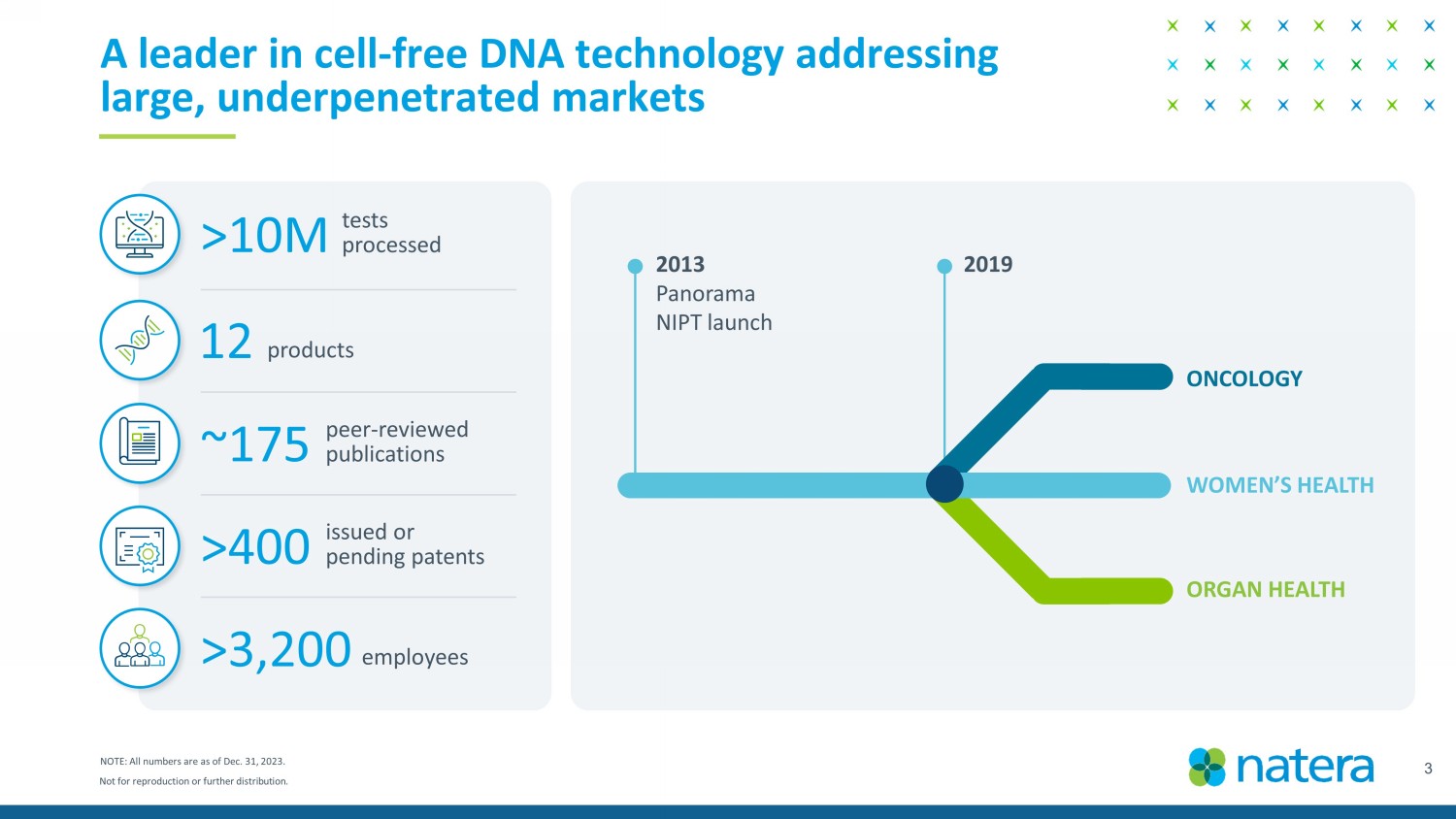
Not for reproduction or further distribution. 3 A leader in cell - free DNA technology addressing large, underpenetrated markets NOTE: All numbers are as of Dec. 31, 2023. ONCOLOGY WOMEN’S HEALTH ORGAN HEALTH 2013 Panorama NIPT launch 2019 12 products >10M tests processed ~ 175 peer - reviewed publications >400 issued or pending patents >3,200 employees
Not for reproduction or further distribution. 4 Formula for success Leading - edge technology + constant innovation Broad and talented commercial teams Excellent customer/ patient experience Leader in peer - reviewed, published clinical data
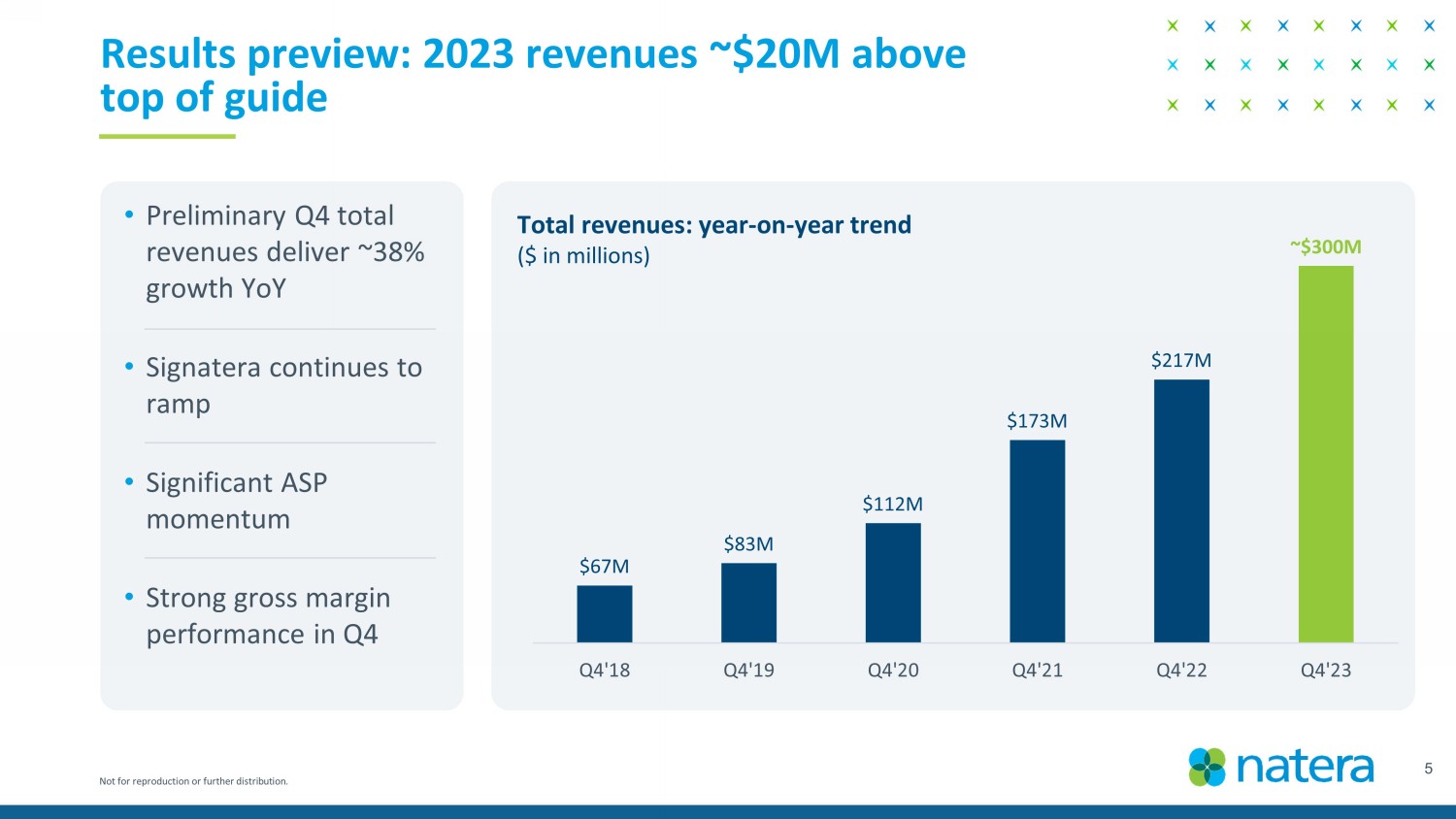
Not for reproduction or further distribution. $67M $83M $112M $173M $217M ~ $300M Q4'18 Q4'19 Q4'20 Q4'21 Q4'22 Q4'23 5 Results preview: 2023 revenues ~$20M above top of guide Total revenues: year - on - year trend ($ in millions) • Preliminary Q4 total revenues deliver ~38% growth YoY • Signatera continues to ramp • Significant ASP momentum • Strong gross margin performance in Q4
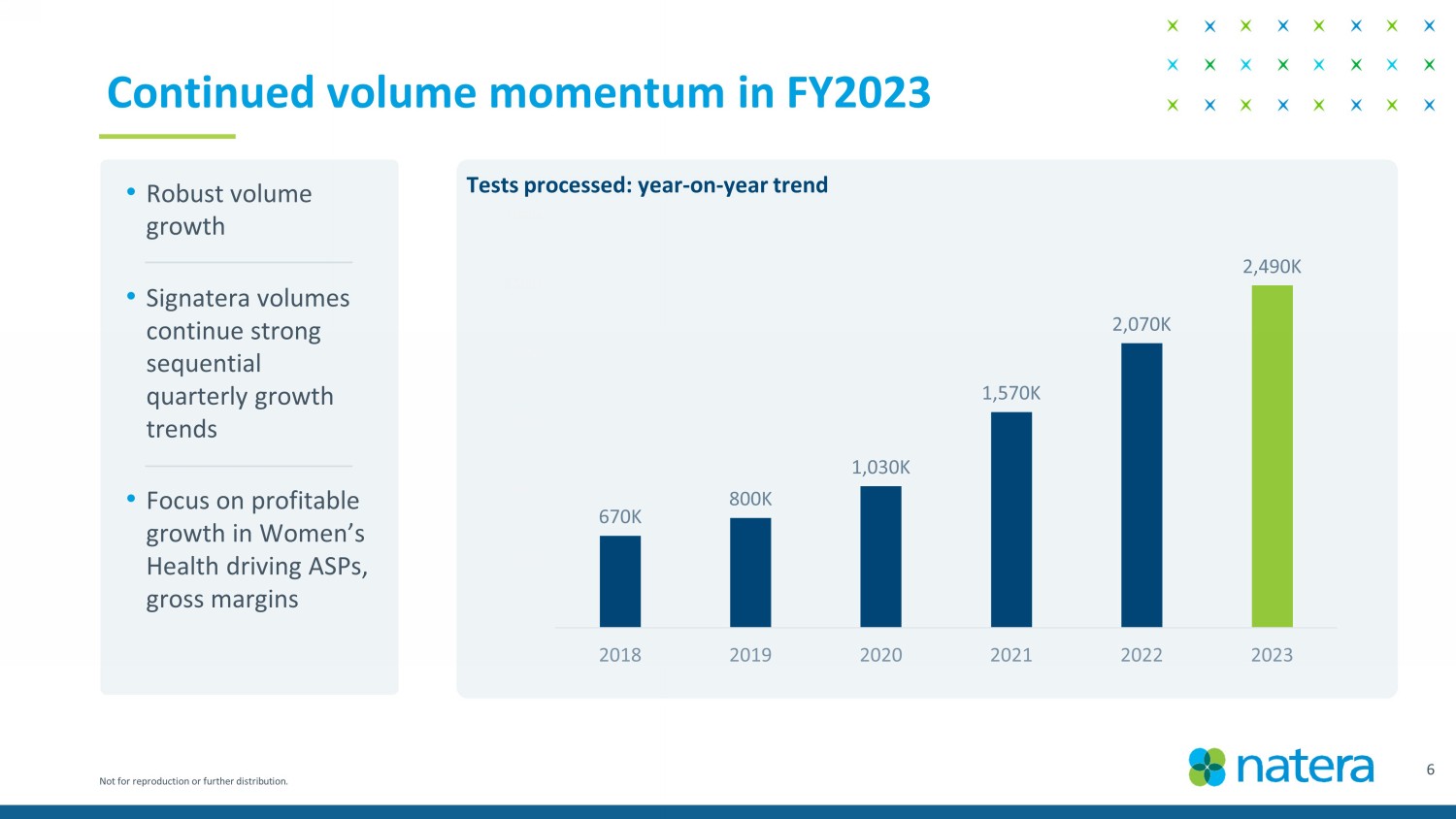
Not for reproduction or further distribution. Continued volume momentum in FY2023 • Robust volume growth • Signatera volumes continue strong sequential quarterly growth trends • Focus on profitable growth in Women’s Health driving ASPs, gross margins 6 670K 800K 1,030K 1,570K 2,070K 2,490K K 500K 1,000K 1,500K 2,000K 2,500K 3,000K 2018 2019 2020 2021 2022 2023 Tests processed : year - on - year trend
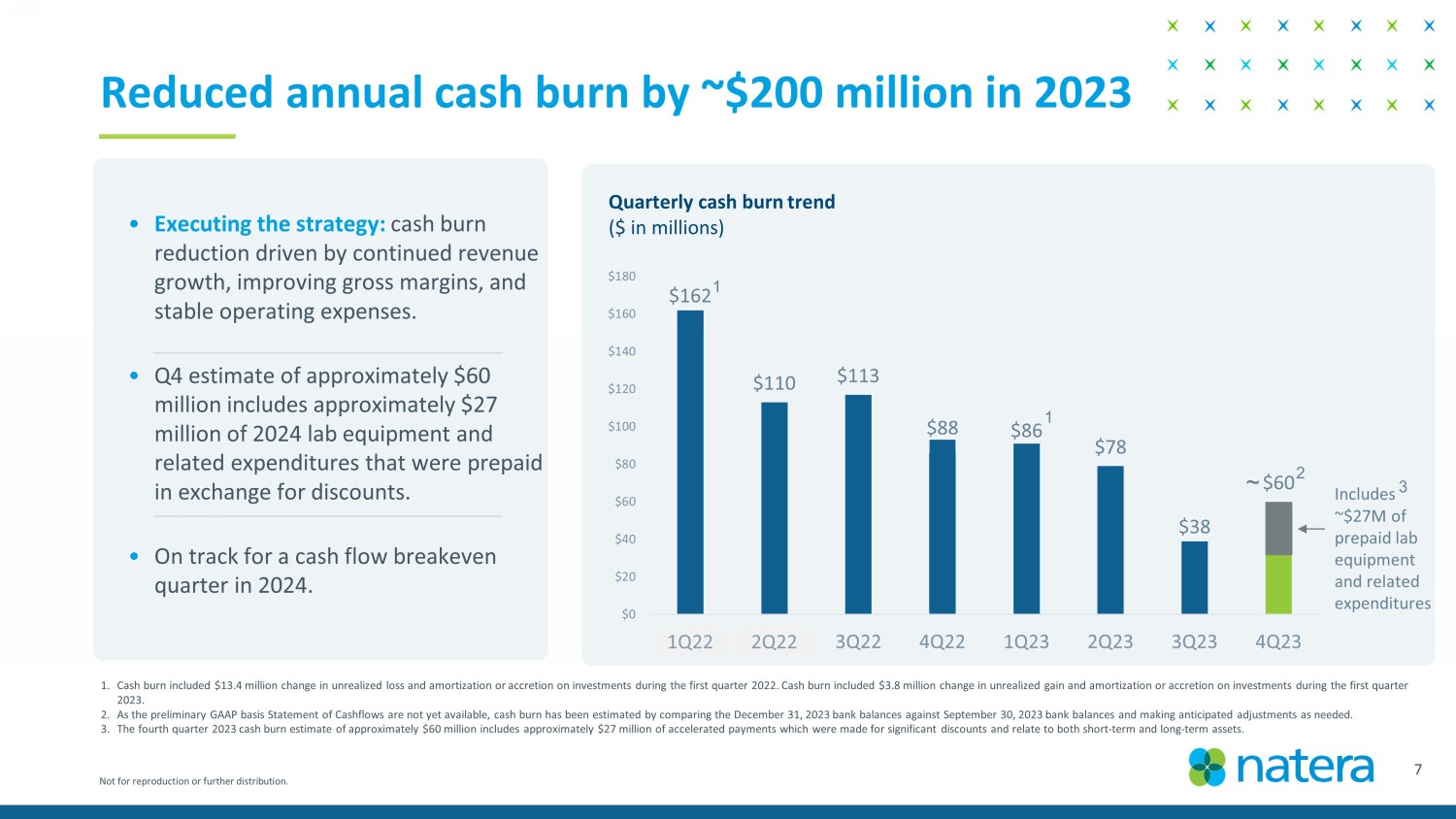
Not for reproduction or further distribution. Reduced annual cash burn by ~$200 million in 2023 1. Cash burn included $13.4 million change in unrealized loss and amortization or accretion on investments during the first quar ter 2022. Cash burn included $3.8 million change in unrealized gain and amortization or accretion on investments during the first q uarter 2023. 2. As the preliminary GAAP basis S tatement of Cashflows are not yet available, c ash burn has been estimated by comparing the December 31, 2023 bank balances against September 30, 2023 bank balances and mak ing anticipated adjustments as needed. 3. The fourth quarter 2023 cash burn estimate of approximately $60 million includes approximately $27 million of accelerated payments which were made for signific ant discounts and relate to both short - term and long - term assets. 7 $162 $110 $113 $88 $86 $78 $38 $60 $0 $20 $40 $60 $80 $100 $120 $140 $160 $180 1Q23 2Q23 3Q22 4Q22 1Q23 2Q23 3Q23 4Q23 Quarterly cash burn trend ($ in millions) ~ 1Q22 2Q22 1 1 2 Includes ~$27M of prepaid lab equipment and related expenditures • Executing the strategy: cash burn reduction driven by continued revenue growth, improving gross margins, and stable operating expenses . • Q4 estimate of approximately $60 million includes approximately $27 million of 2024 lab equipment and related expenditures that were prepaid in exchange for discounts. • On track for a cash flow breakeven quarter in 2024. 3

Not for reproduction or further distribution. 8 Market leadership in oncology MRD NOTE: 35% of oncologists ordered Natera’s tests as of Sept. 30, 2023. All other numbers are as of Dec. 31, 2023. >60 peer - reviewed studies >3 0 0K of oncologists ordered Natera’s tests 35% tests performed in 2023 >150K patients in our clinical - genomic database > 400 commercial operations and medical affairs employees $20B estimated TAM
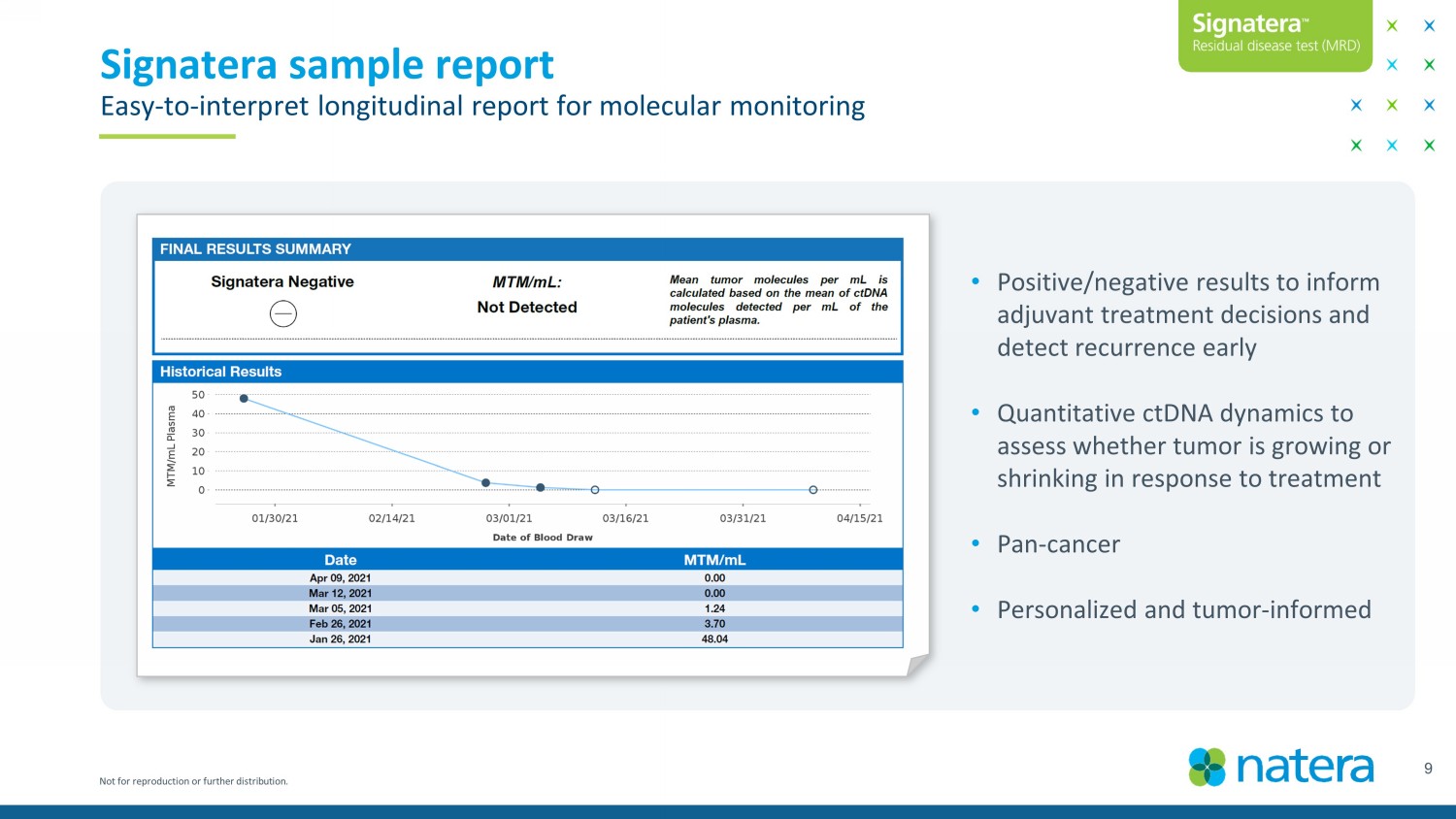
Not for reproduction or further distribution. Signatera sample report Easy - to - interpret longitudinal report for molecular monitoring 9 • Positive/negative results to inform adjuvant treatment decisions and detect recurrence early • Quantitative ctDNA dynamics to assess whether tumor is growing or shrinking in response to treatment • Pan - cancer • Personalized and tumor - informed
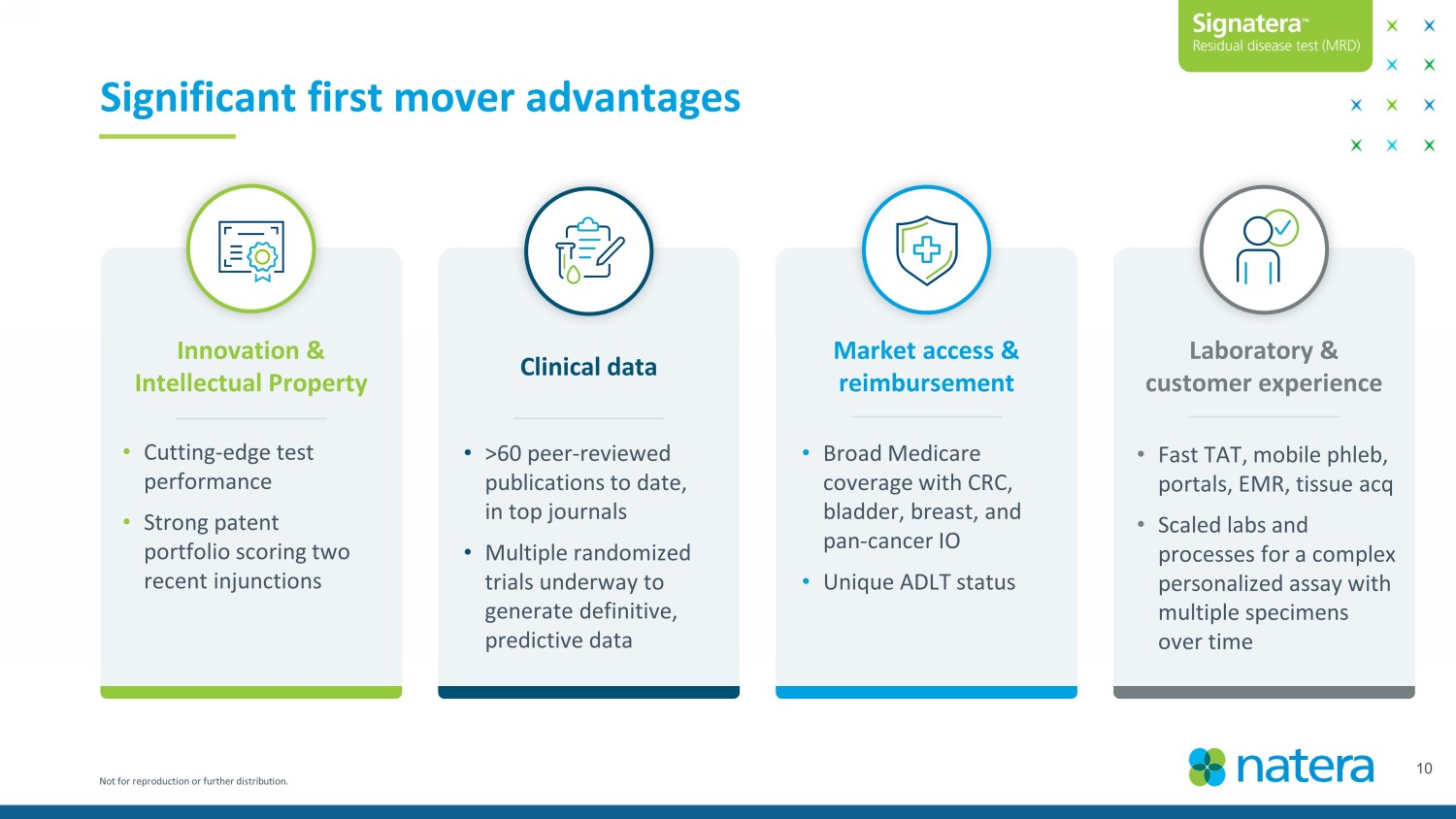
Not for reproduction or further distribution. 10 Significant first mover advantages Innovation & Intellectual Property Clinical data Market access & reimbursement Laboratory & customer experience • Cutting - edge test performance • Strong patent portfolio scoring two recent injunctions • >60 peer - reviewed publications to date, in top journals • Multiple randomized trials underway to generate definitive, predictive data • Broad Medicare coverage with CRC, bladder, breast, and pan - cancer IO • Unique ADLT status • Fast TAT, mobile phleb , portals, EMR, tissue acq • Scaled labs and processes for a complex personalized assay with multiple specimens over time
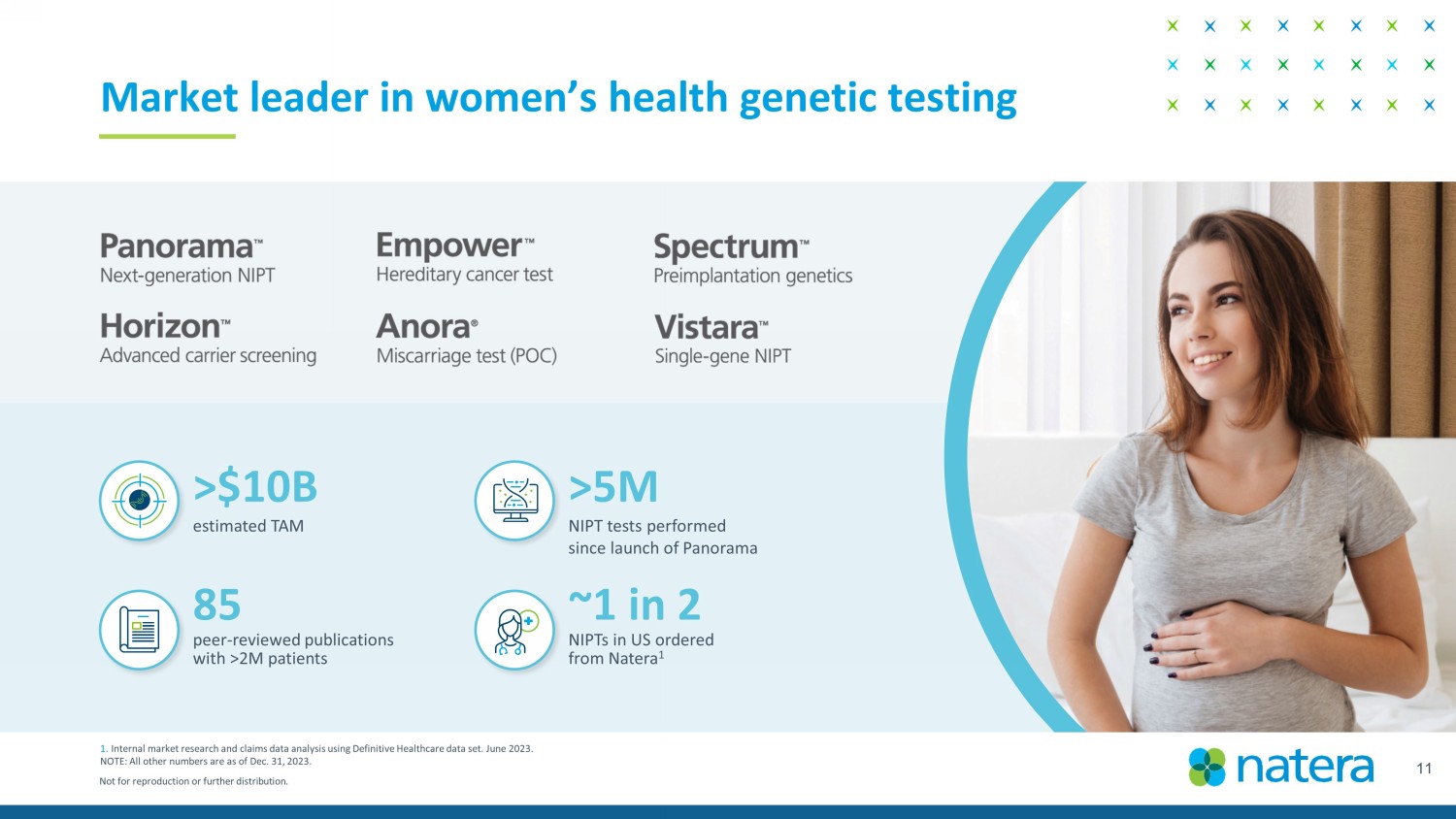
Not for reproduction or further distribution. 11 Market leader in women’s health genetic testing 1. Internal market research and claims data analysis using Definitive Healthcare data set. June 2023. NOTE: All other numbers are as of Dec. 31, 2023. >5M NIPT tests performed since launch of Panorama 85 peer - reviewed publications with >2M patients ~1 in 2 NIPTs in US ordered from Natera 1 >$10B estimated TAM
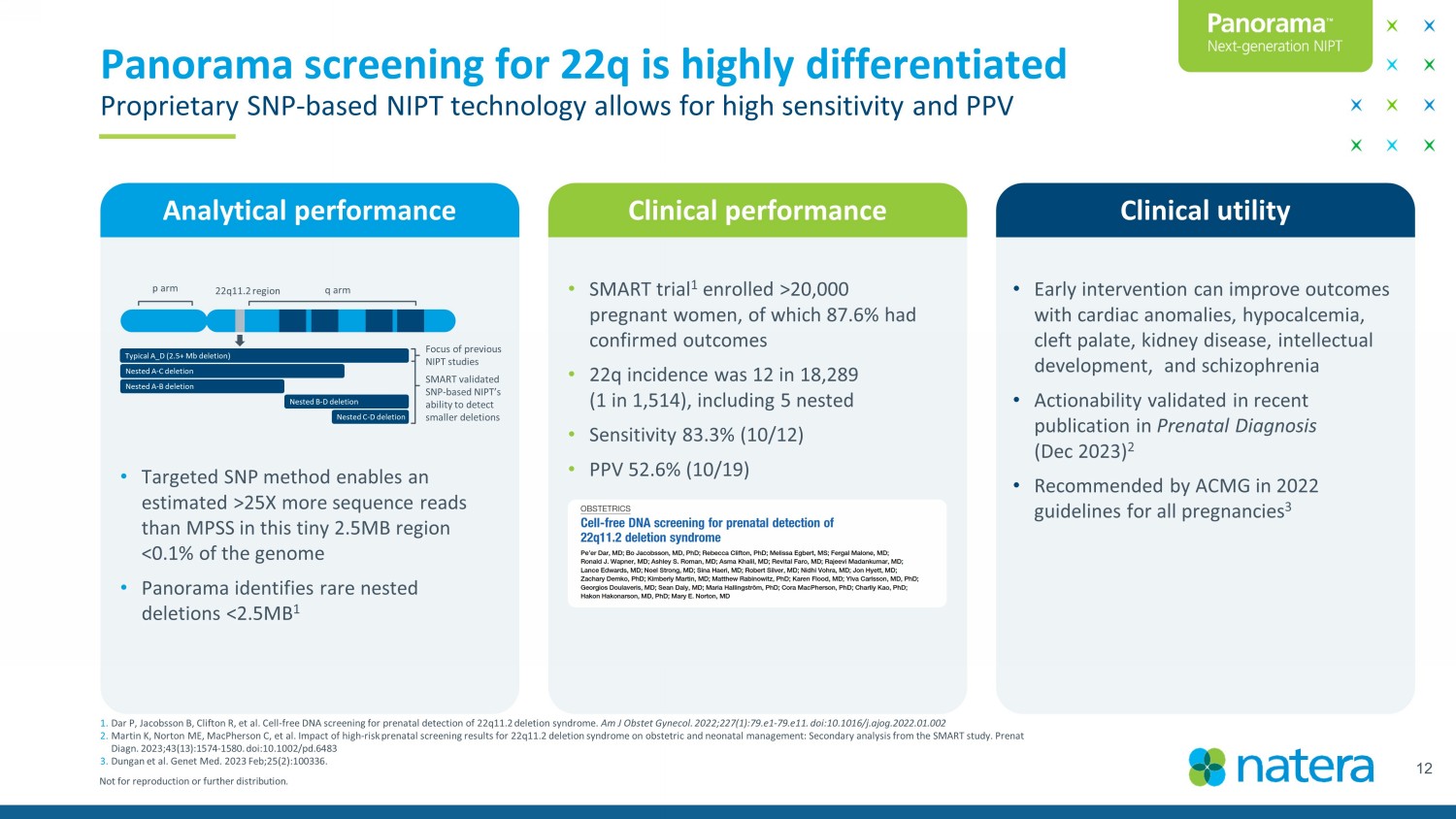
Not for reproduction or further distribution. Analytical performance Clinical performance • SMART trial 1 enrolled >20,000 pregnant women, of which 87.6% had confirmed outcomes • 22q incidence was 12 in 18,289 (1 in 1,514), including 5 nested • Sensitivity 83.3% (10/12) • PPV 52.6% (10/19) Clinical utility 12 Panorama screening for 22q is highly differentiated Proprietary SNP - based NIPT technology allows for high sensitivity and PPV 1. Dar P, Jacobsson B, Clifton R, et al. Cell - free DNA screening for prenatal detection of 22q11.2 deletion syndrome. Am J Obstet Gynecol. 2022;227(1):79.e1 - 79.e11. doi:10.1016/j.ajog.2022.01.002 2. Martin K, Norton ME, MacPherson C, et al. Impact of high - risk prenatal screening results for 22q11.2 deletion syndrome on obstet ric and neonatal management: Secondary analysis from the SMART study. Prenat Diagn . 2023;43(13):1574 - 1580. doi:10.1002/pd.6483 3. Dungan et al. Genet Med. 2023 Feb;25(2):100336. • Targeted SNP method enables an estimated >25X more sequence reads than MPSS in this tiny 2.5MB region <0.1% of the genome • Panorama identifies rare nested deletions <2.5MB 1 • Early intervention can improve outcomes with cardiac anomalies, hypocalcemia, cleft palate, kidney disease, intellectual development, and schizophrenia • Actionability validated in recent publication in Prenatal Diagnosis (Dec 2023) 2 • Recommended by ACMG in 2022 guidelines for all pregnancies 3 p arm q arm 22q11.2 region Focus of previous NIPT studies SMART validated SNP - based NIPT’s ability to detect smaller deletions Typical A_D (2.5+ Mb deletion) Nested A - C deletion Nested A - B deletion Nested B - D deletion Nested C - D deletion
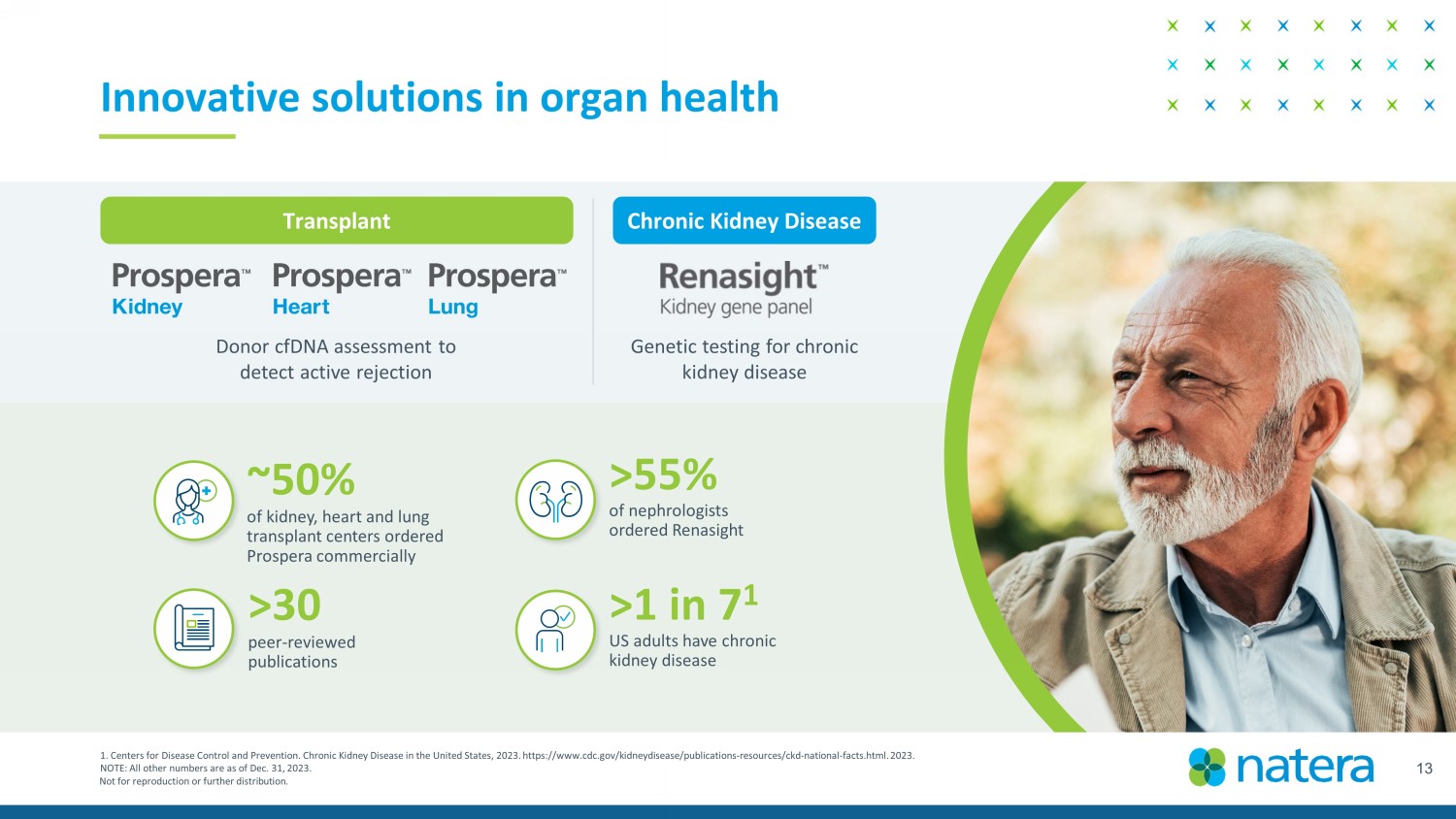
Not for reproduction or further distribution. 13 Innovative solutions in organ health 1. Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2023. https://www.cdc.gov/kidneyd ise ase/publications - resources/ckd - national - facts.html. 2023. NOTE: All other numbers are as of Dec. 31, 2023. >55% of nephrologists ordered Renasight >1 in 7 1 US adults have chronic kidney disease ~ 50% o f kidney, heart and lung transplant centers ordered Prospera commercially Transplant Chronic Kidney Disease Donor cfDNA assessment to detect active rejection Genetic testing for chronic kidney disease >30 peer - reviewed publications
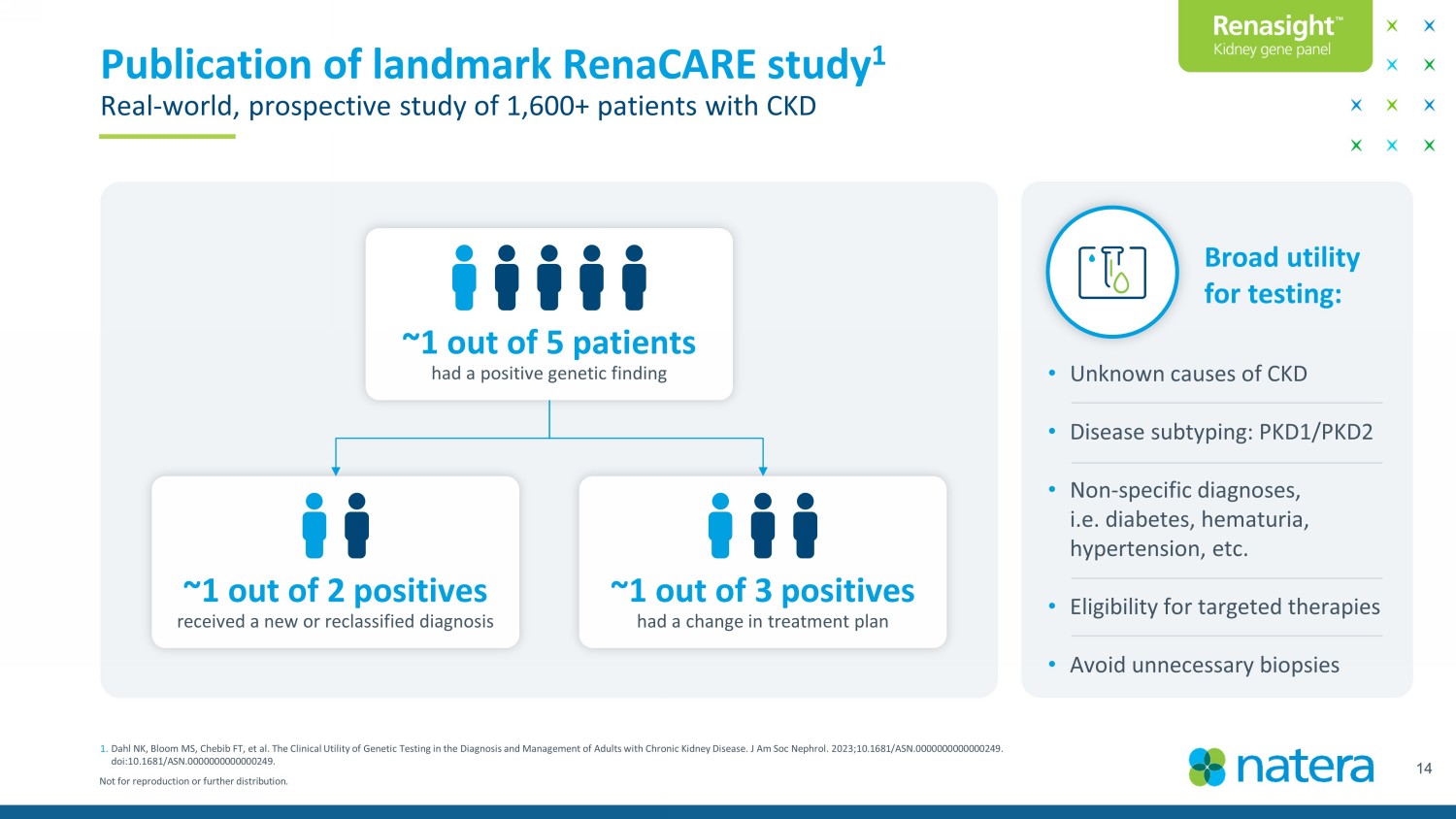
Not for reproduction or further distribution. 14 Publication of landmark RenaCARE study 1 Real - world, prospective study of 1,600+ patients with CKD 1. Dahl NK, Bloom MS, Chebib FT, et al. The Clinical Utility of Genetic Testing in the Diagnosis and Management of Adults with Chronic Kidney Disease. J Am Soc Nephrol. 2023;10.1681/ASN.0000000000000249. doi:10.1681/ASN.0000000000000249. • Unknown causes of CKD • Disease subtyping: PKD1/PKD2 • Non - specific diagnoses, i.e. diabetes, hematuria, hypertension, etc. • Eligibility for targeted therapies • Avoid unnecessary biopsies Broad utility for testing: ~1 out of 5 patients had a positive genetic finding ~1 out of 3 positives had a change in treatment plan ~1 out of 2 positives received a new or reclassified diagnosis

Not for reproduction or further distribution. 15 Upcoming potential catalysts Natera today Large potential catalysts • Strong revenue growth driven by volumes and ASPs • On track for cash flow break even quarter in 2024 regardless of any catalysts • Established lab and commercial footprint • Significant historical investments in innovation coming to fruition • Guideline inclusion of women’s health products • Readout of multiple randomized trials, including ALTAIR in 2024 • Commercial coverage for Signatera and Prospera • Exciting new product launches and enhancements in core business and beyond

©202 4 Natera, Inc. All Rights Reserved. Not for reproduction or further distribution. ® 16