| NEUBASE THERAPEUTICS, INC. The Future of Gene Editing is Stealth Editing™ Investor Conference Call | May 22, 2023 |
| Safe Harbor Statement Certain statements contained in this presentation regarding matters that are not historical facts are forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, known as the PSLRA. These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. NeuBase Therapeutics, Inc. (“NeuBase”) undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. NeuBase uses words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future ,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” the negative of these terms, and similar expressions to identify these forward-looking statements that are intended to be covered by the safe harbor provisions of the PSLRA. Such forward-looking statements are based on NeuBase’s expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, NeuBase’s plans to research, develop and commercialize any product candidates; the timing of initiation of any clinical trials; the risk that prior data will not be replicated in future studies; the timing of any investigational new drug application or new drug application; the clinical utility, potential benefits and market acceptance of any product candidates; NeuBase’s commercialization, marketing and manufacturing capabilities and strategy; global health conditions, including the impact of COVID-19; NeuBase’s ability to protect its intellectual property position; and the requirement for additional capital to continue to advance NeuBase’s product candidates, which may not be available on favorable terms or at all. New factors emerge from time to time and it is not possible for NeuBase to predict all such factors, nor can NeuBase assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements included in this presentation are based on information available to NeuBase as of the date of this presentation. NeuBase disclaims any obligation to update such forward-looking statements to reflect events or circumstances after the date of this presentation, except as required by applicable law. This presentation does not constitute an offer to sell, or the solicitation of an offer to buy, any securities. This presentation may contain trademarks, service marks, trade names and copyrights of third parties, which are the property of their respective owners. The use or display of third parties' trademarks, service marks, trade names or products in this presentation is not intended to, and does not imply, a relationship with NeuBase or an endorsement or sponsorship by or of NeuBase. Solely for convenience, the trademarks, service marks, trade names and copyrights referred to in this presentation may appear without the TM, SM, * or © symbols, but such references are not intended to indicate, in any way, that NeuBase will not assert, to the fullest extent under applicable law, their rights or right of the applicable licensor to these trademarks, service marks, trade names or copyrights. All rights reserved. 2 |
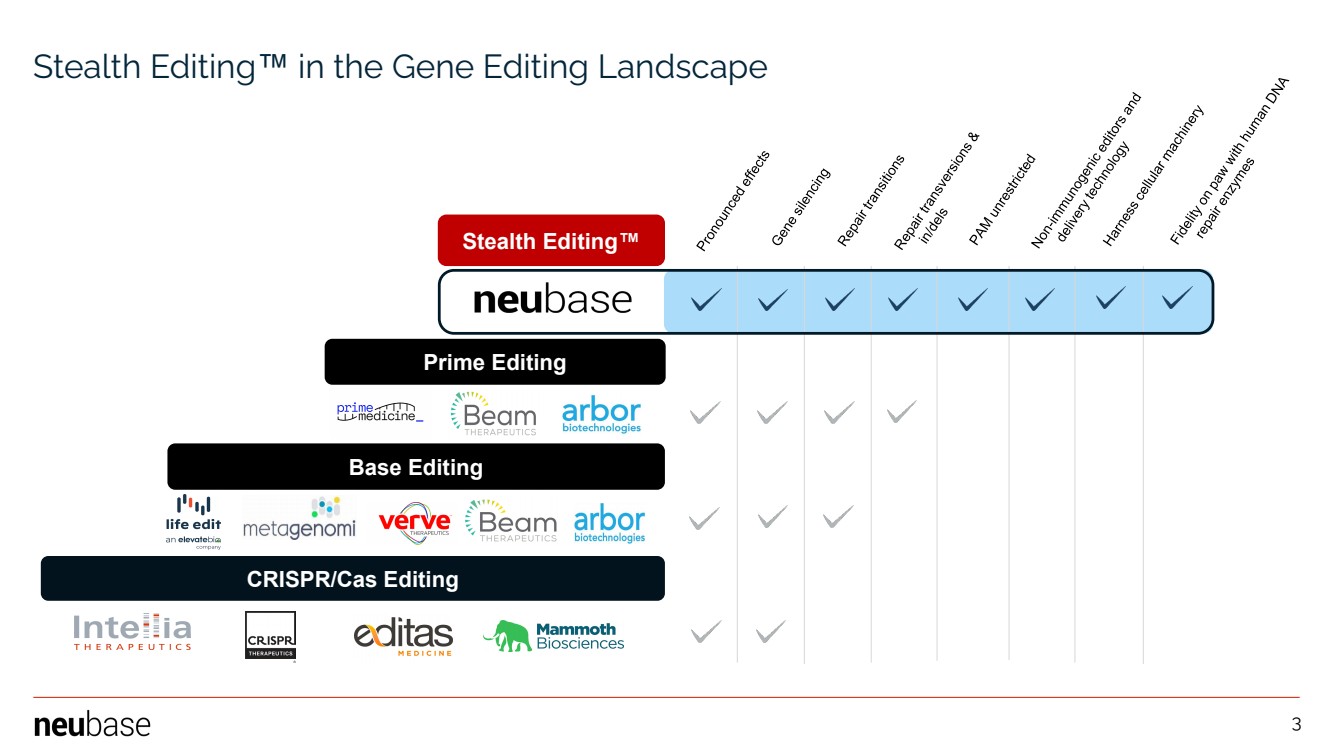
| NEUBASE THERAPEUTICS, INC. 3 Stealth Editing™ Prime Editing Base Editing CRISPR/Cas Editing Stealth Editing™ in the Gene Editing Landscape 3 |
| NEUBASE THERAPEUTICS, INC. 4 Stealth Editors™ - designed to fly under the radar of the immune system to effect high fidelity gene editing Immunogenicity from viral delivery systems and bacterial proteins presents a potential safety issue for patients receiving gene editing therapies 4 |
| NEUBASE THERAPEUTICS, INC. 5 Why Stealth Editors™ Non-viral Delivery With a non-immunogenic system that can reach diverse tissues and cell types via systemic administration Pronounced In Vivo Effects Through the resolution of pathogenesis in a titratable and re-dosable fashion Broadly Applicable Can achieve gene disruption/repair across multiple species and industries Harnesses Cellular Mechanisms Through the utilization of DNA repair machinery that has been refined over millennia to deliver precise on-target edits with minimal off-target edits 5 |
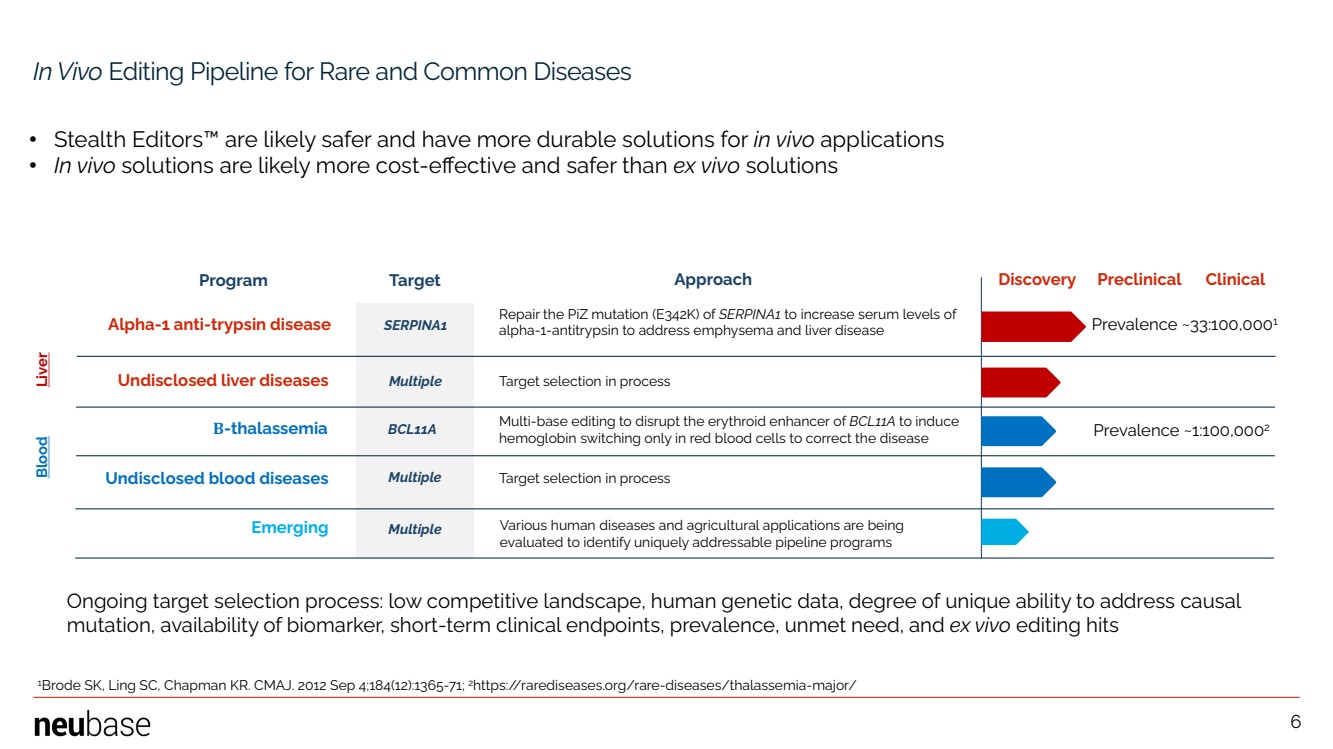
| NEUBASE THERAPEUTICS, INC. 6 In Vivo Editing Pipeline for Rare and Common Diseases Program Target Approach Discovery Preclinical Clinical Alpha-1 anti-trypsin disease SERPINA1 BCL11A Repair the PiZ mutation (E342K) of SERPINA1 to increase serum levels of alpha-1-antitrypsin to address emphysema and liver disease Multiple Multiple Multiple 6 Emerging Undisclosed blood diseases Various human diseases and agricultural applications are being evaluated to identify uniquely addressable pipeline programs • Stealth Editors™ are likely safer and have more durable solutions for in vivo applications • In vivo solutions are likely more cost-effective and safer than ex vivo solutions Undisclosed liver diseases Β-thalassemia Target selection in process Multi-base editing to disrupt the erythroid enhancer of BCL11A to induce hemoglobin switching only in red blood cells to correct the disease Target selection in process Ongoing target selection process: low competitive landscape, human genetic data, degree of unique ability to address causal mutation, availability of biomarker, short-term clinical endpoints, prevalence, unmet need, and ex vivo editing hits Liver Blood Prevalence ~33:100,0001 Prevalence ~1:100,0002 1 Brode SK, Ling SC, Chapman KR. CMAJ. 2012 Sep 4;184(12):1365-71; 2https://rarediseases.org/rare-diseases/thalassemia-major/ |
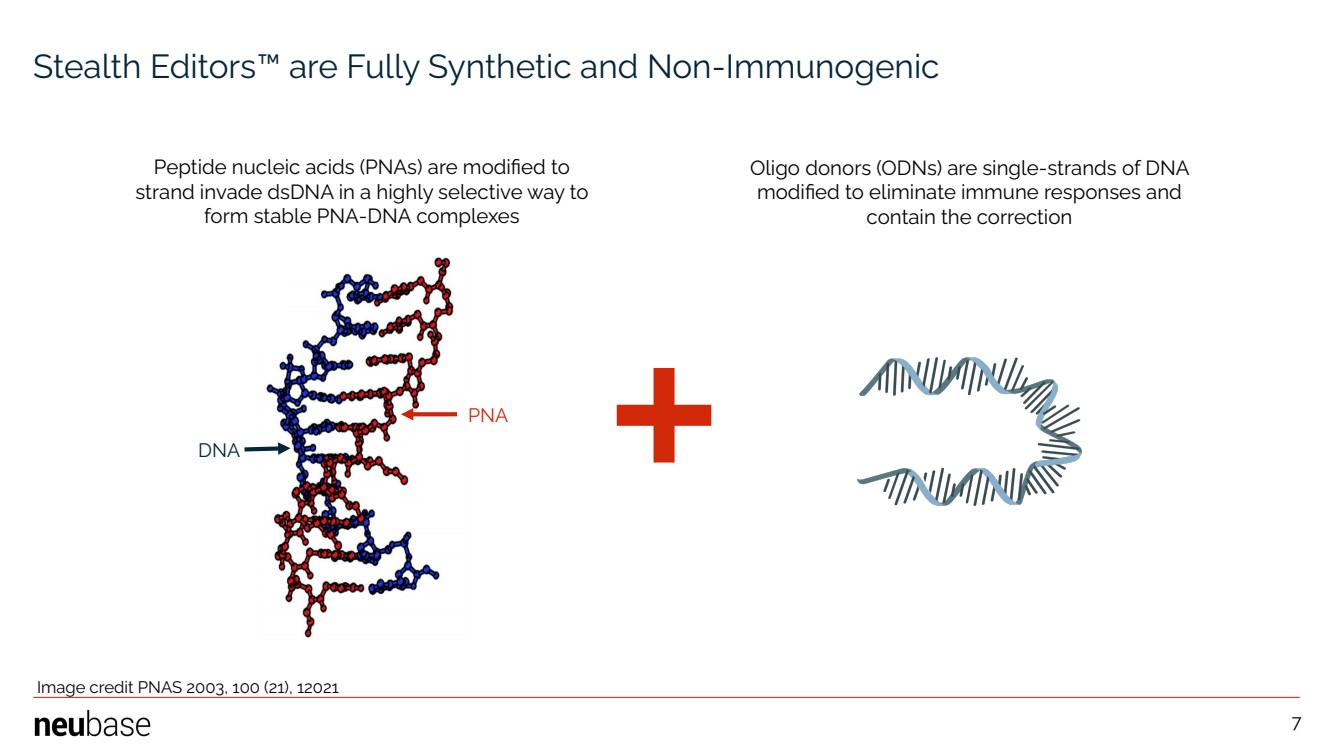
| NEUBASE THERAPEUTICS, INC. 7 Stealth Editors™ are Fully Synthetic and Non-Immunogenic Peptide nucleic acids (PNAs) are modified to strand invade dsDNA in a highly selective way to form stable PNA-DNA complexes Image credit PNAS 2003, 100 (21), 12021 PNA DNA Oligo donors (ODNs) are single-strands of DNA modified to eliminate immune responses and contain the correction 7 |
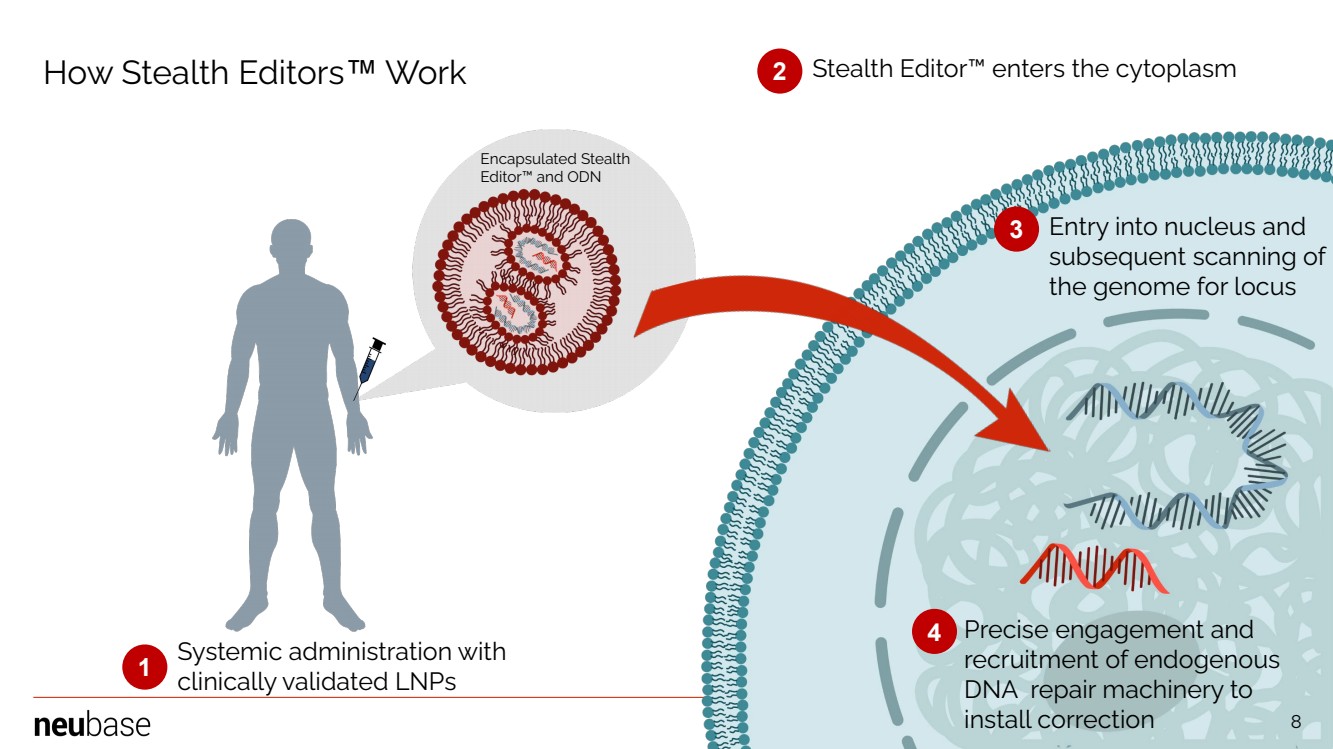
| NEUBASE THERAPEUTICS, INC. 8 Systemic administration with clinically validated LNPs Stealth Editor™ enters the cytoplasm Entry into nucleus and subsequent scanning of the genome for locus Precise engagement and recruitment of endogenous DNA repair machinery to install correction 1 2 3 4 Encapsulated Stealth Editor™ and ODN How Stealth Editors™ Work 8 |
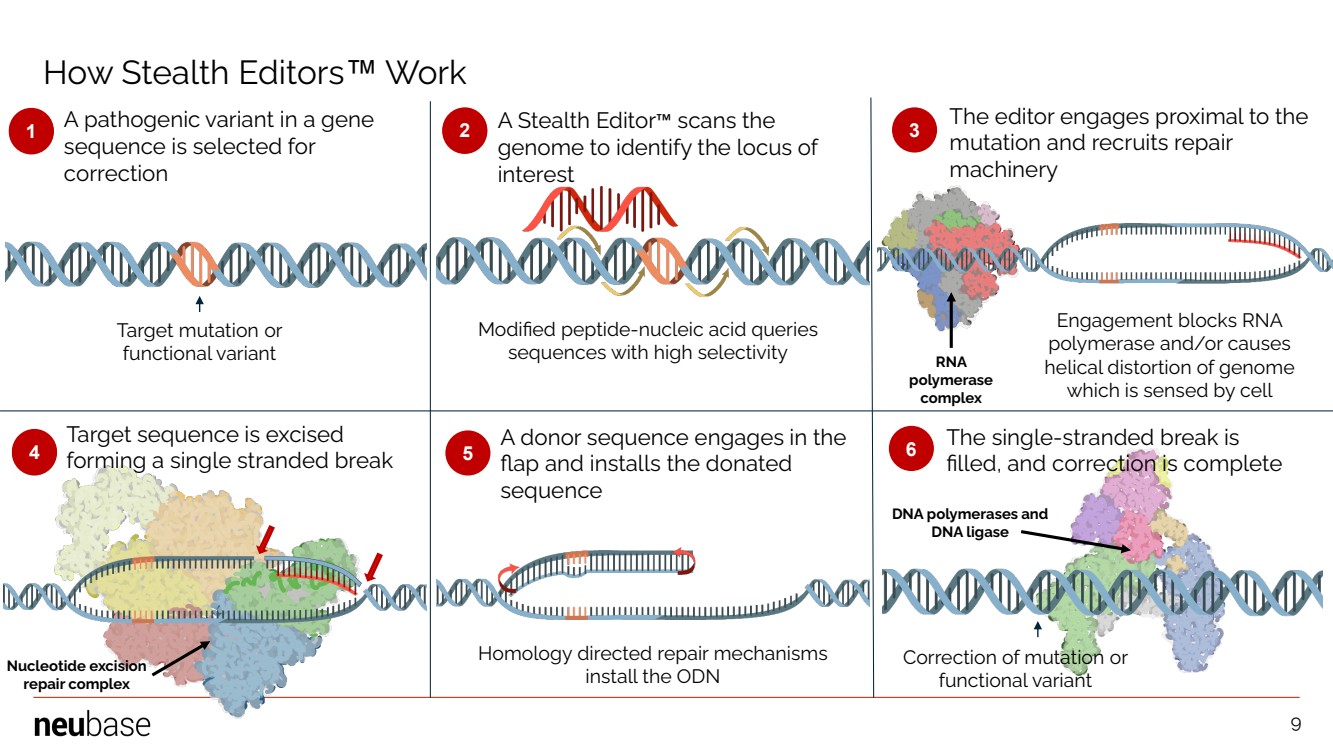
| NEUBASE THERAPEUTICS, INC. 9 1 2 4 5 6 3 4 A pathogenic variant in a gene sequence is selected for correction A Stealth Editor™ scans the genome to identify the locus of interest The editor engages proximal to the mutation and recruits repair machinery Target sequence is excised forming a single stranded break A donor sequence engages in the flap and installs the donated sequence The single-stranded break is filled, and correction is complete Target mutation or functional variant Modified peptide-nucleic acid queries sequences with high selectivity Engagement blocks RNA polymerase and/or causes helical distortion of genome which is sensed by cell Correction of mutation or functional variant DNA polymerases and DNA ligase Nucleotide excision repair complex RNA polymerase complex Homology directed repair mechanisms install the ODN How Stealth Editors™ Work 9 |
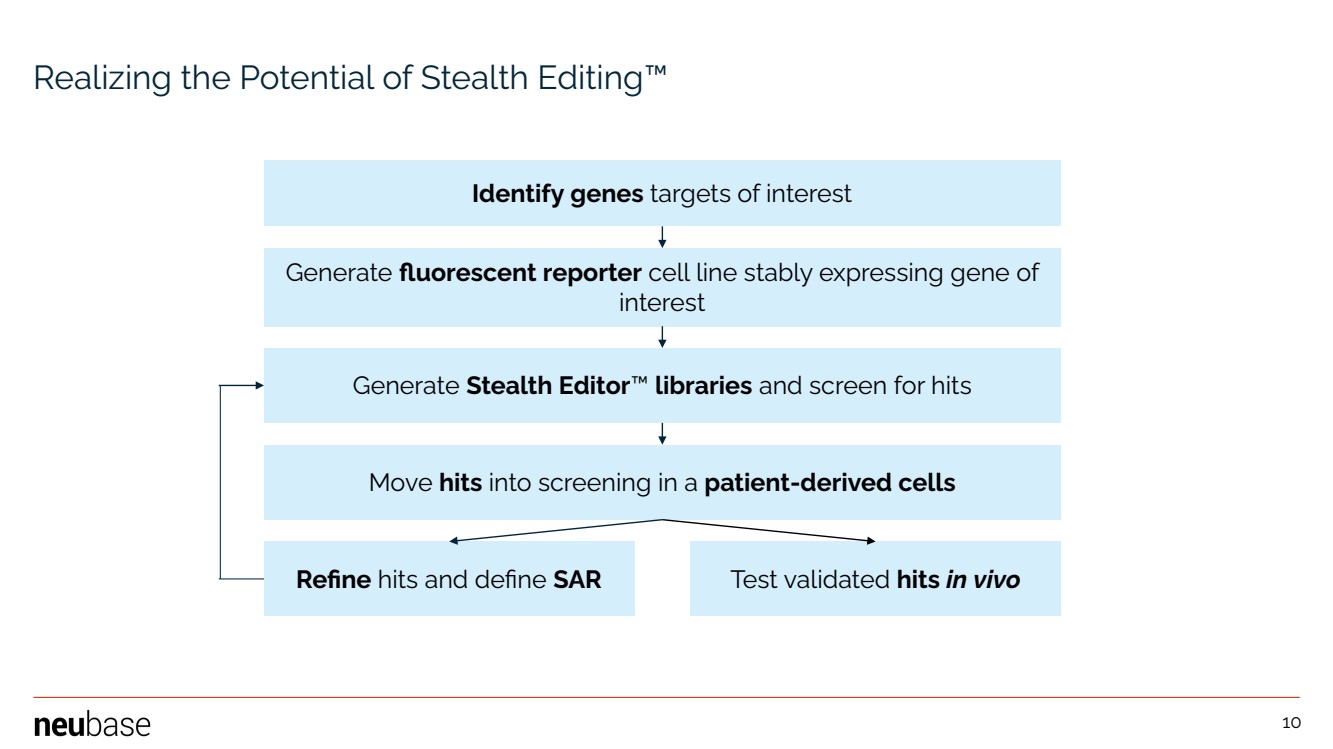
| NEUBASE THERAPEUTICS, INC. 10 Realizing the Potential of Stealth Editing™ Identify genes targets of interest Generate fluorescent reporter cell line stably expressing gene of interest Generate Stealth Editor™ libraries and screen for hits Move hits into screening in a patient-derived cells Refine hits and define SAR Test validated hits in vivo 10 |
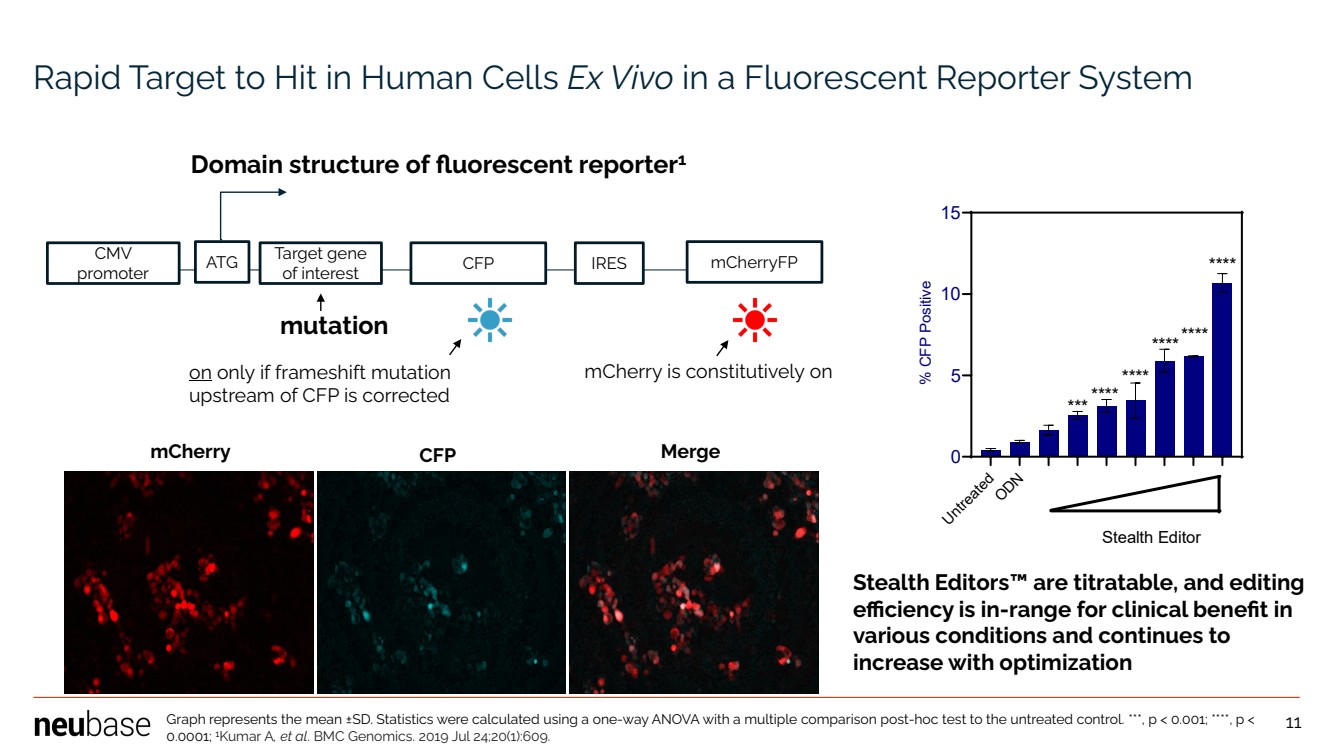
| NEUBASE THERAPEUTICS, INC. 11 mCherry CFP Merge Domain structure of fluorescent reporter1 CMV promoter ATG Target gene of interest CFP IRES mCherryFP on only if frameshift mutation upstream of CFP is corrected mutation mCherry is constitutively on Rapid Target to Hit in Human Cells Ex Vivo in a Fluorescent Reporter System Stealth Editors™ are titratable, and editing efficiency is in-range for clinical benefit in various conditions and continues to increase with optimization Untreated ODN 0 5 10 15 % CFP Positive ******* **** **** **** **** Stealth Editor Graph represents the mean ±SD. Statistics were calculated using a one-way ANOVA with a multiple comparison post-hoc test to the untreated control. ***, p < 0.001; ****, p < 0.0001; 1 Kumar A, et al. BMC Genomics. 2019 Jul 24;20(1):609. 11 |
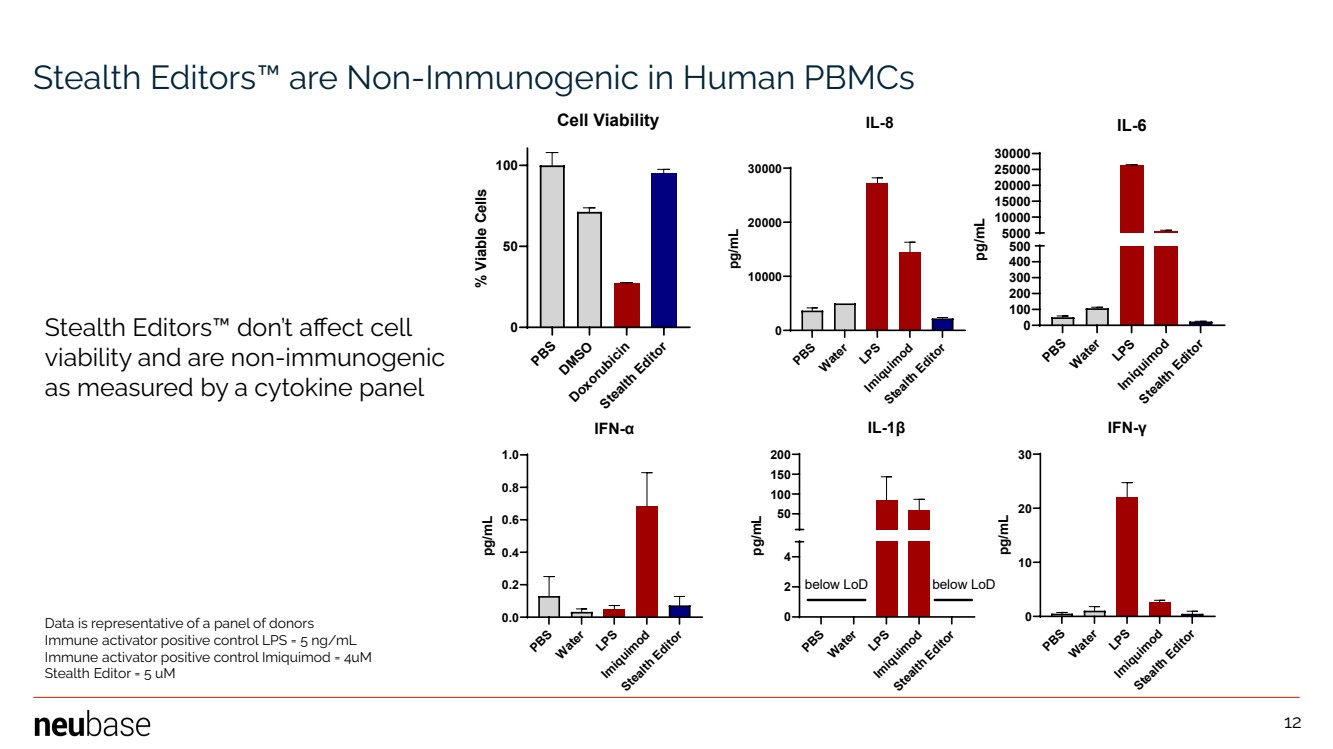
| NEUBASE THERAPEUTICS, INC. 12 Stealth Editors™ are Non-Immunogenic in Human PBMCs PBS Water LPS Imiquimod Stealth Editor 0 10000 20000 30000 IL-8 pg/mL PBS Water LPS Imiquimod Stealth Editor 0 100 200 300 400 500 5000 10000 15000 20000 25000 30000 IL-6 pg/mL PBS Water LPS Imiquimod Stealth Editor 0.0 0.2 0.4 0.6 0.8 1.0 IFN-α pg/mL PBS Water LPS Imiquimod Stealth Editor 0 2 4 50 100 150 200 IL-1β pg/mL below LoD below LoD PBS Water LPS Imiquimod Stealth Editor 0 10 20 30 IFN-γ pg/mL PBS DMSO Doxorubicin Stealth Editor 0 50 100 Cell Viability % Viable Cells Stealth Editors™ don’t affect cell viability and are non-immunogenic as measured by a cytokine panel Data is representative of a panel of donors Immune activator positive control LPS = 5 ng/mL Immune activator positive control Imiquimod = 4uM Stealth Editor = 5 uM 12 |
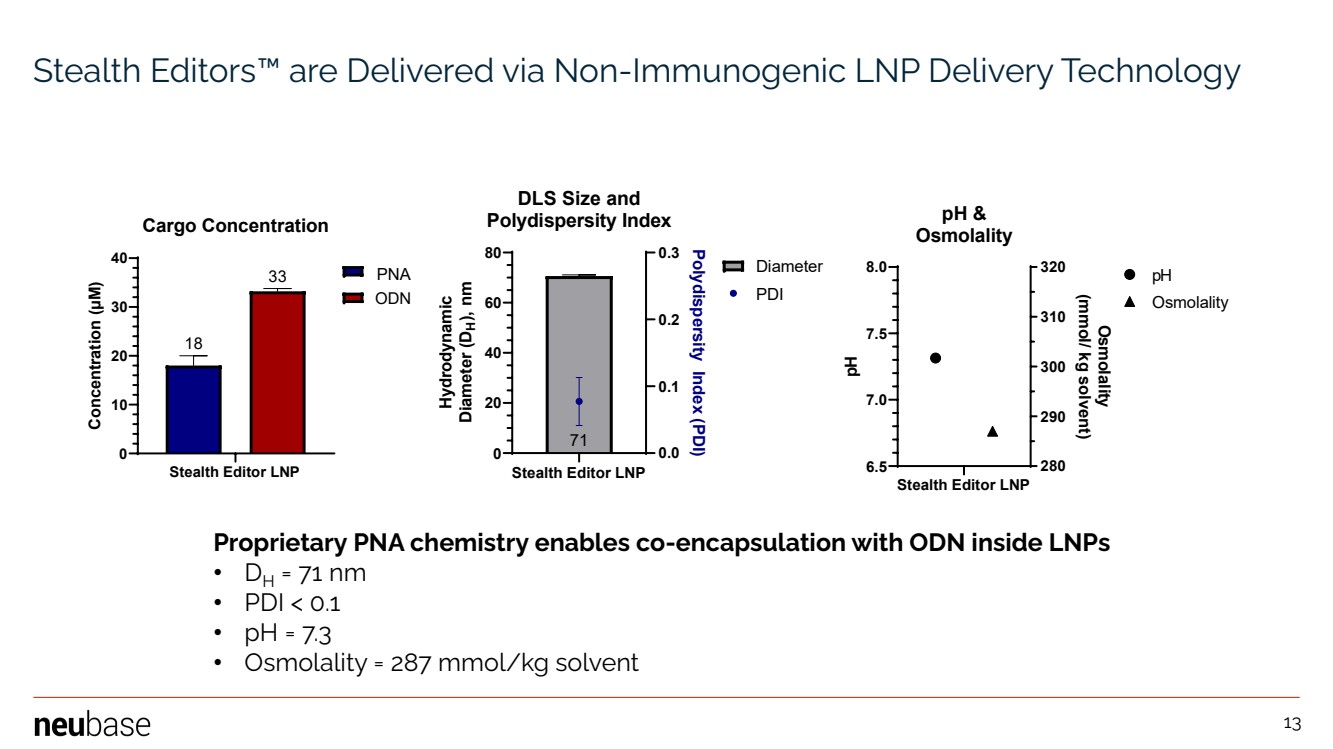
| NEUBASE THERAPEUTICS, INC. 13 Stealth Editors™ are Delivered via Non-Immunogenic LNP Delivery Technology Stealth Editor LNP 6.5 7.0 7.5 8.0 280 290 300 310 320 pH & Osmolality pH Osmolality (mmol/ kg solvent) Osmolality pH Stealth Editor LNP 0 10 20 30 40 33 18 Cargo Concentration Concentration ( μM) 951.H ODN Stealth Editor LNP 0 20 40 60 80 0.0 0.1 0.2 0.3 71 DLS Size and Polydispersity Index Hydrodynamic Diameter (D H), nm Polydispersity Index (PDI) Diameter PDI PNA Proprietary PNA chemistry enables co-encapsulation with ODN inside LNPs • DH = 71 nm • PDI < 0.1 • pH = 7.3 • Osmolality = 287 mmol/kg solvent 13 |
| NEUBASE THERAPEUTICS, INC. 14 www.neubasetherapeutics.com Investor contact: Dan Ferry (daniel@lifesciadvisors.com) |