
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s
telephone number, including area code:
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 2.02 Results of Operations and Financial Condition
On January 9, 2023, Anebulo Pharmaceuticals, Inc. (the “Company”) announced in its corporate presentation that as of December 31, 2022 it had $16 million in cash which is expected to provide a cash runway into 2024.
The information in this Item 2.02 of this Current Report on 8-K is furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information shall not be deemed incorporated by reference into any other filing with the Securities and Exchange Commission made by the Company, whether made before or after today’s date, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific references in such filing.
Item 7.01 Regulation FD Disclosure.
On January 9, 2023, the Company updated its corporate slide presentation for use in meetings with investors, analysts and others. The presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference herein.
The information in this Item 7.01 of this Current Report on 8-K (including Exhibit 99.1) is furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information shall not be deemed incorporated by reference into any other filing with the Securities and Exchange Commission made by the Company, whether made before or after today’s date, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific references in such filing.
Item 8.01 Other Events.
On January 9, 2023, the Company issued a press release titled, “Anebulo Pharmaceuticals Announces Completion of Dosing and Preliminary Data from Part B of its Phase 2 Study of ANEB-001 for Acute Cannabinoid Intoxication.” The press release is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit Number |
Description | |
| 99.1 | Company Presentation dated January 2023 | |
| 99.2 | Press Release of Anebulo Pharmaceuticals, Inc., dated January 9, 2023 | |
| 104 | Cover Page of Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ANEBULO PHARMACEUTICALS, INC. | ||
| Date: January 9, 2023 | By: | /s/ Simon Allen |
| Simon Allen | ||
| Chief Executive Officer | ||
Exhibit 99.1



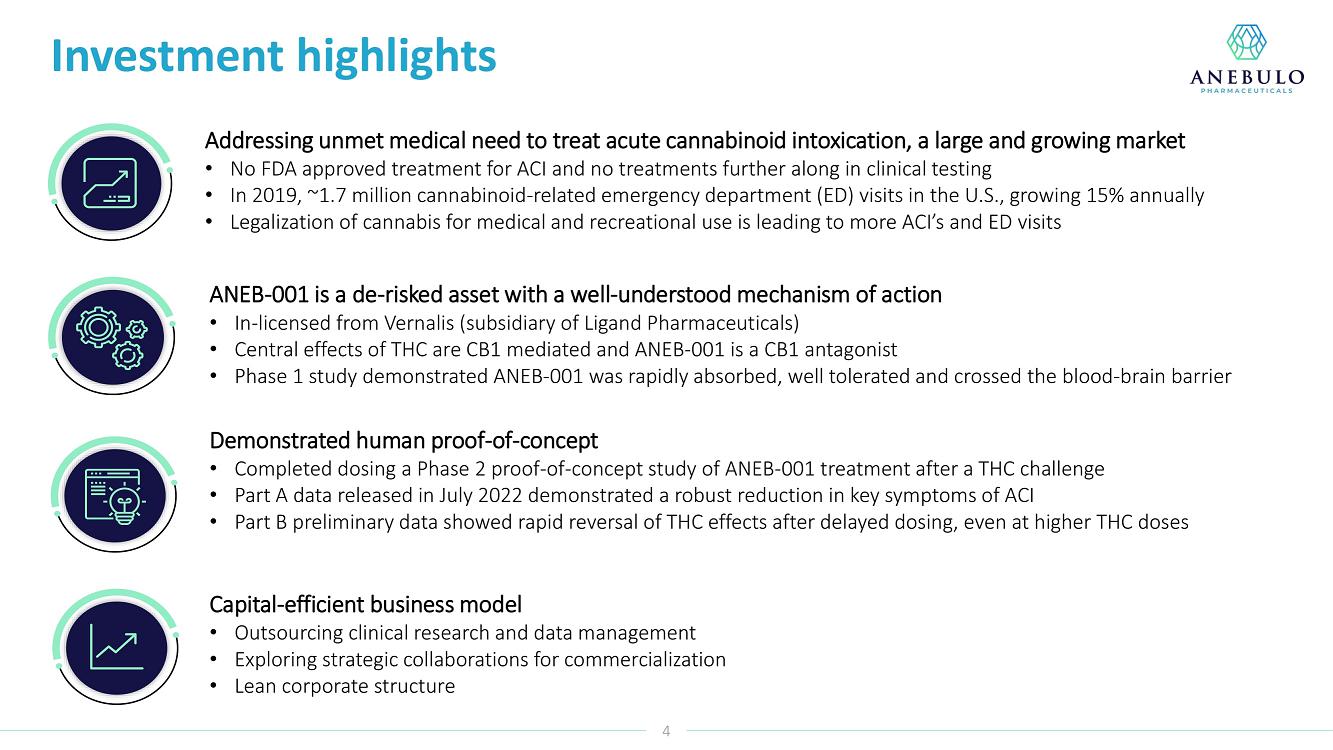



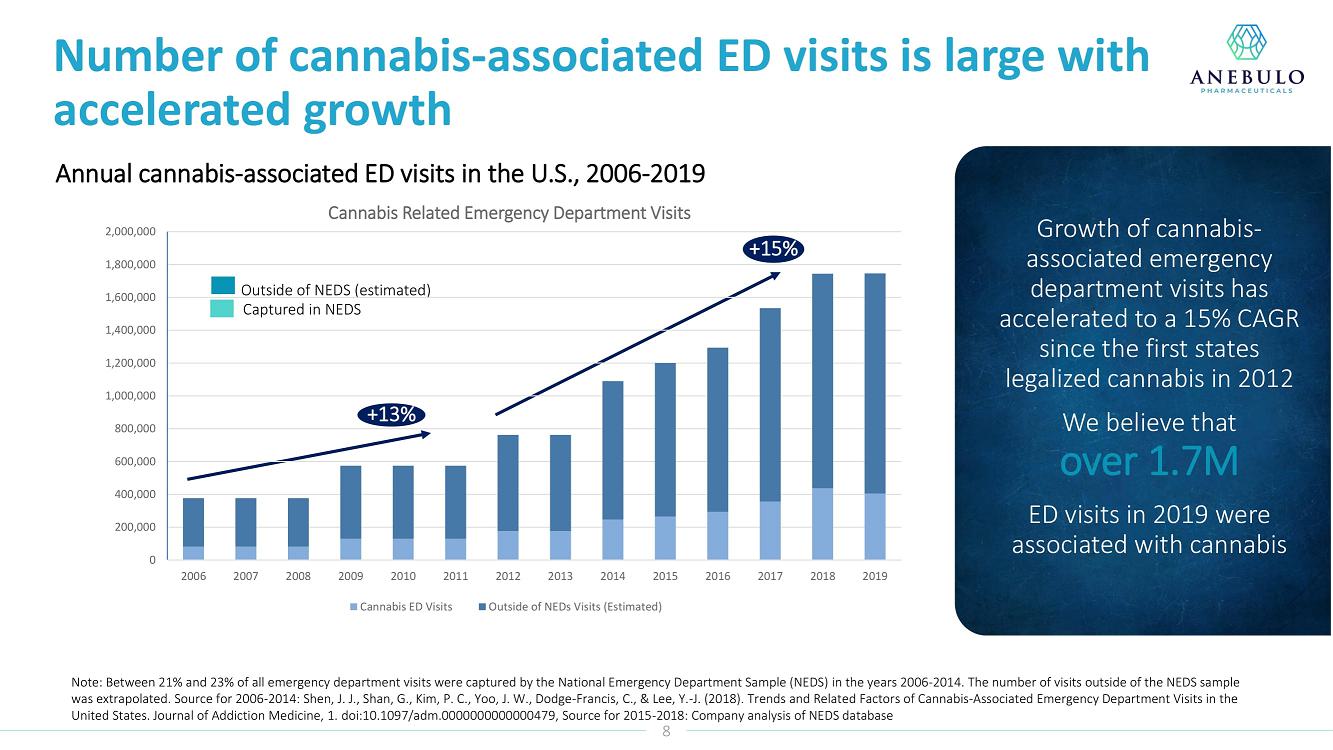
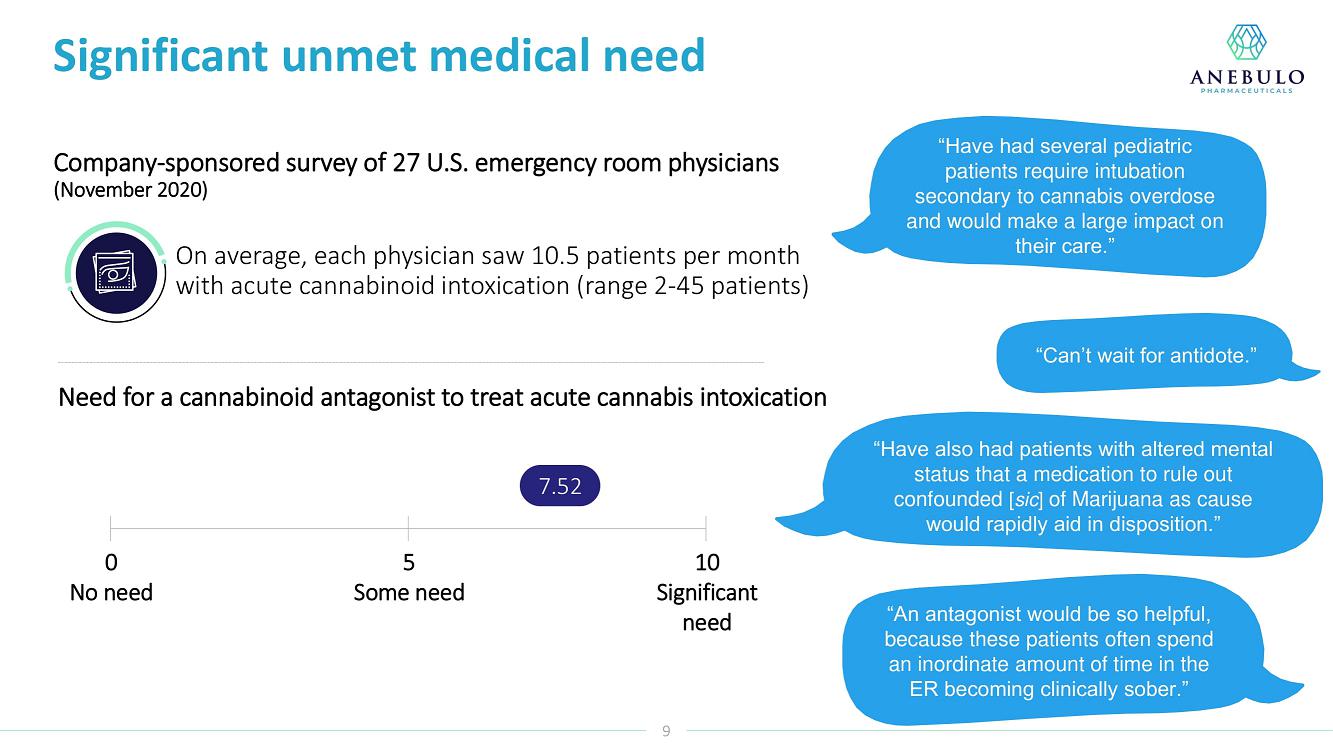
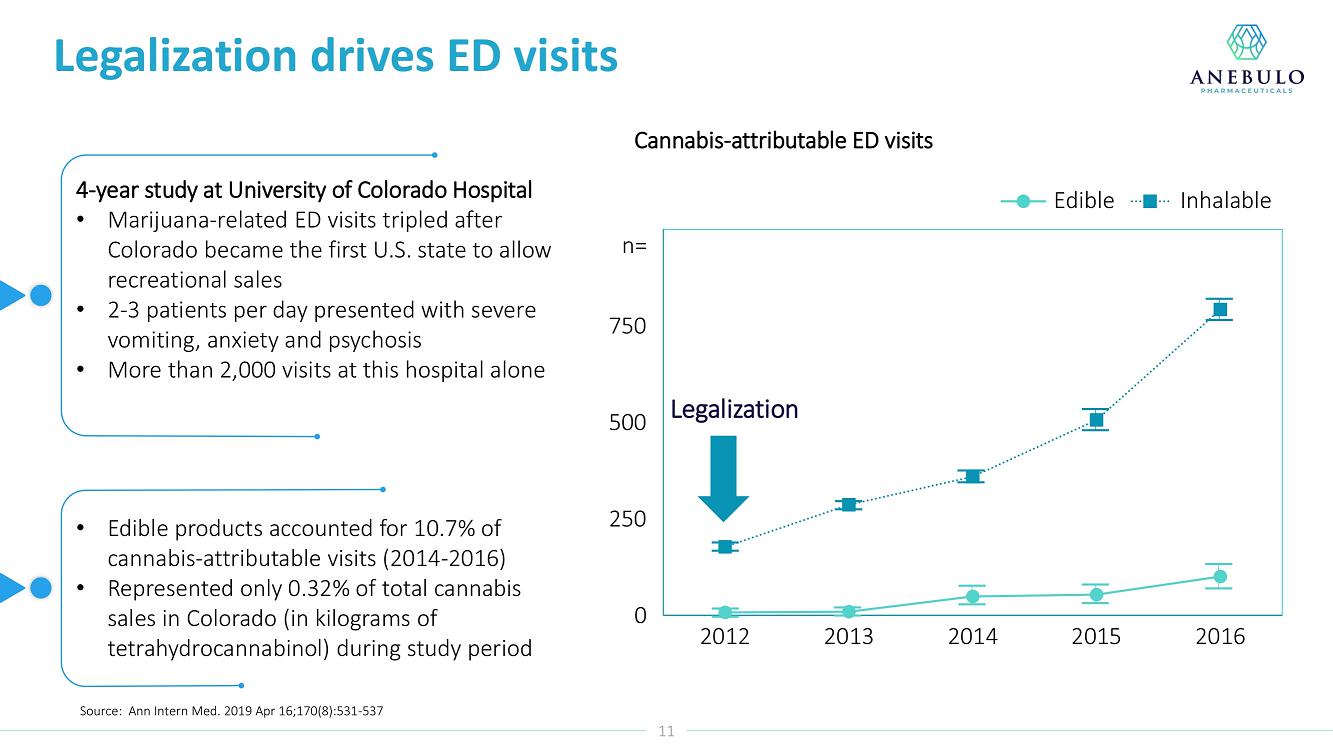
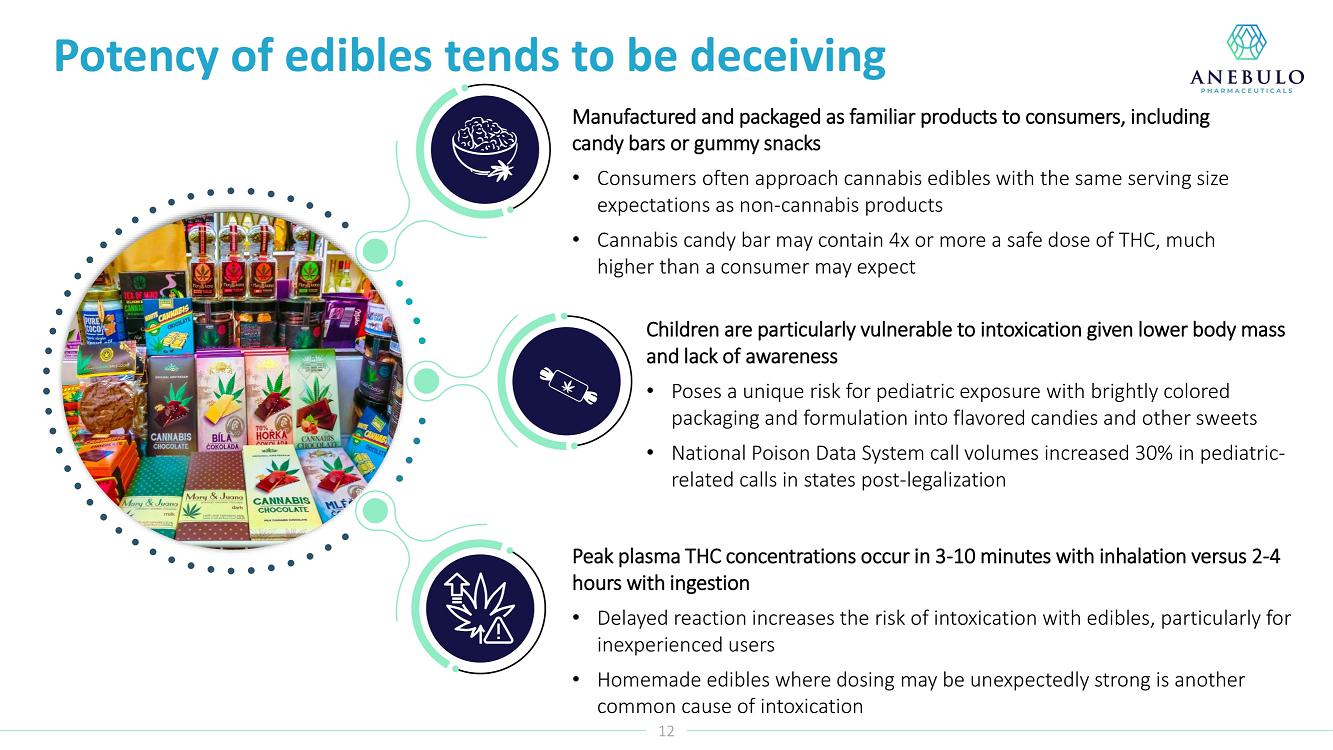



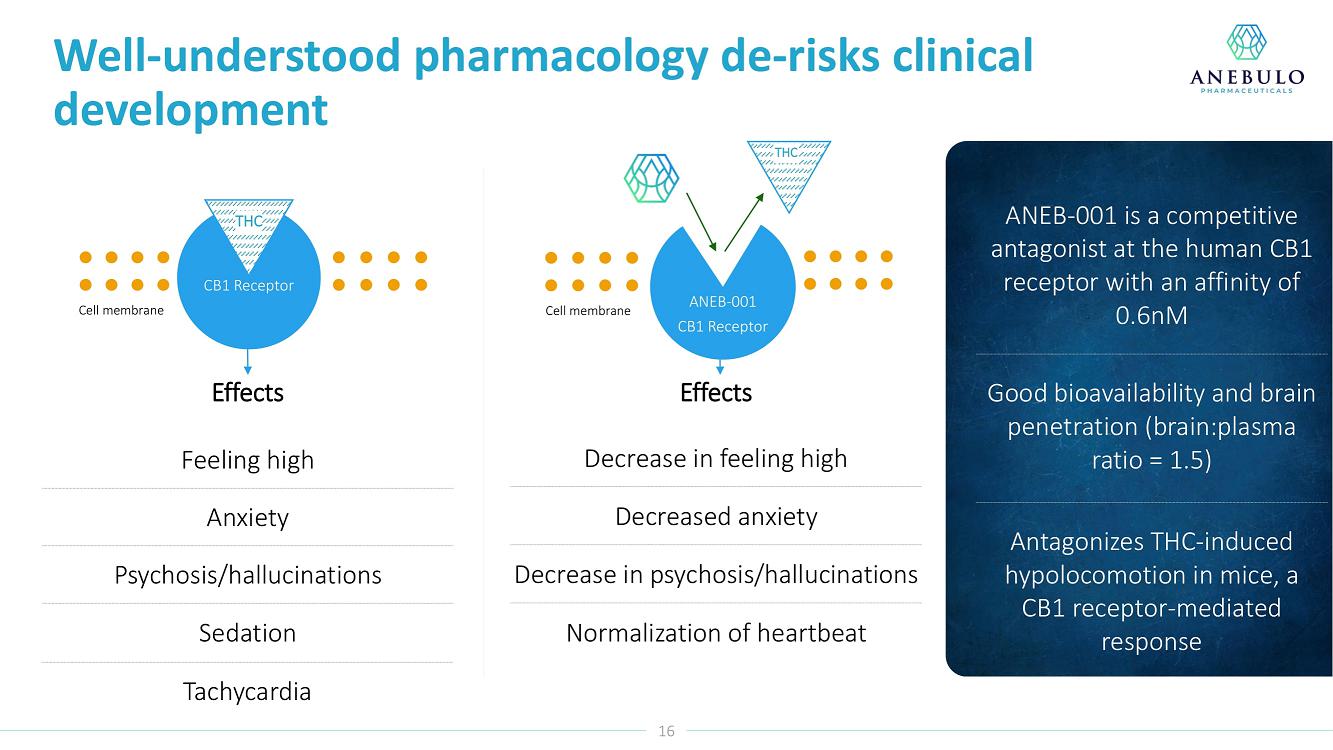

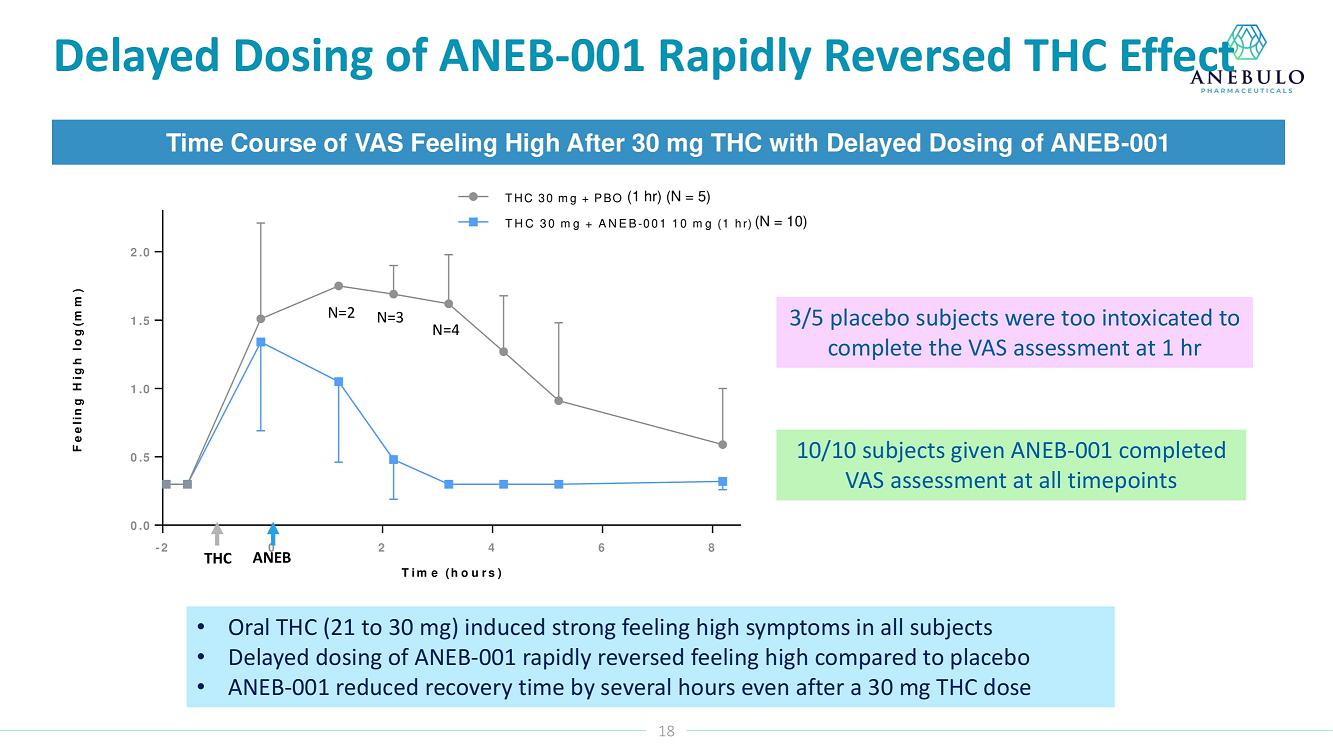

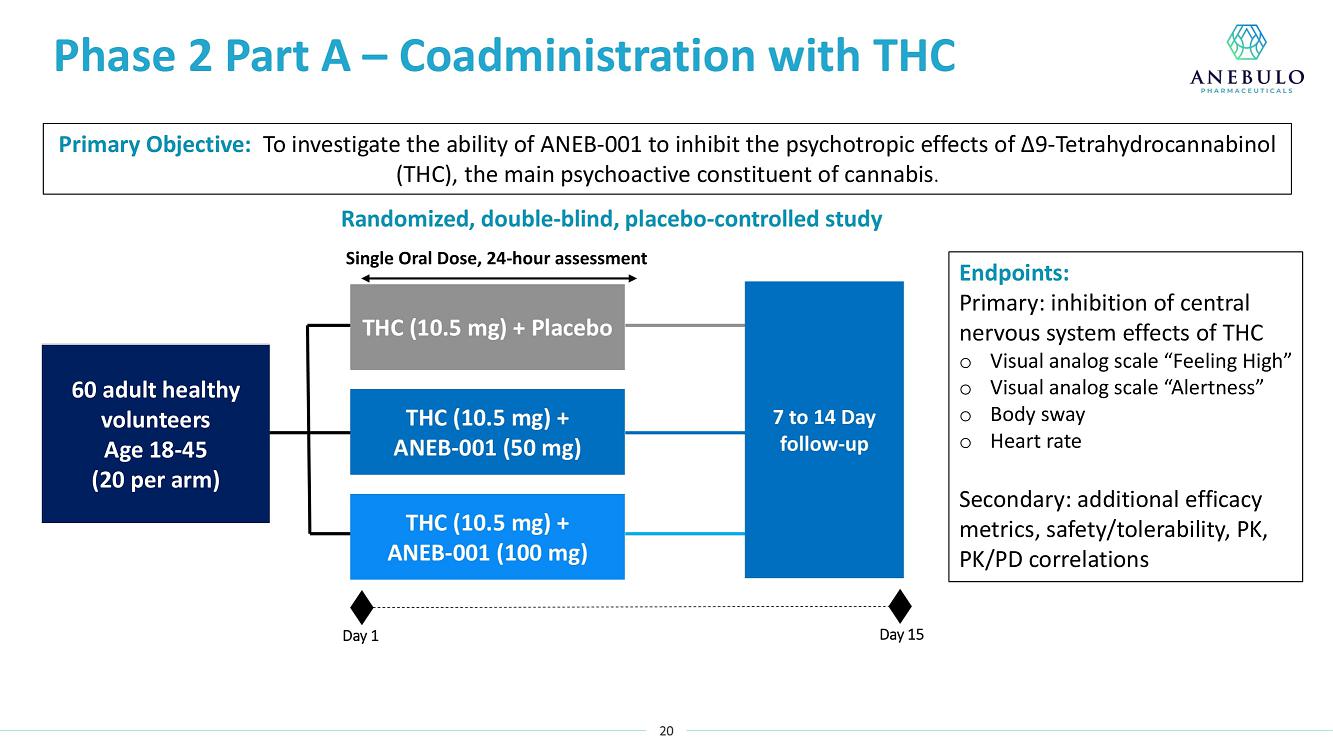
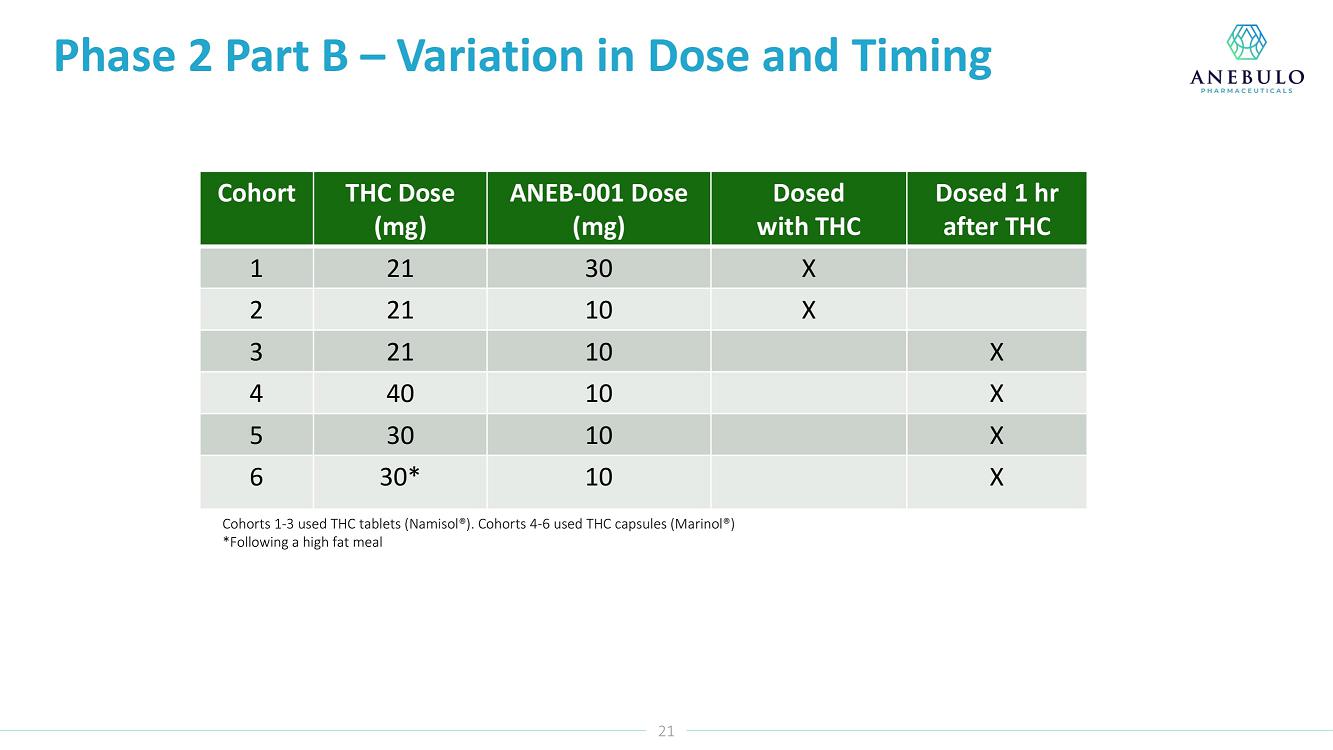
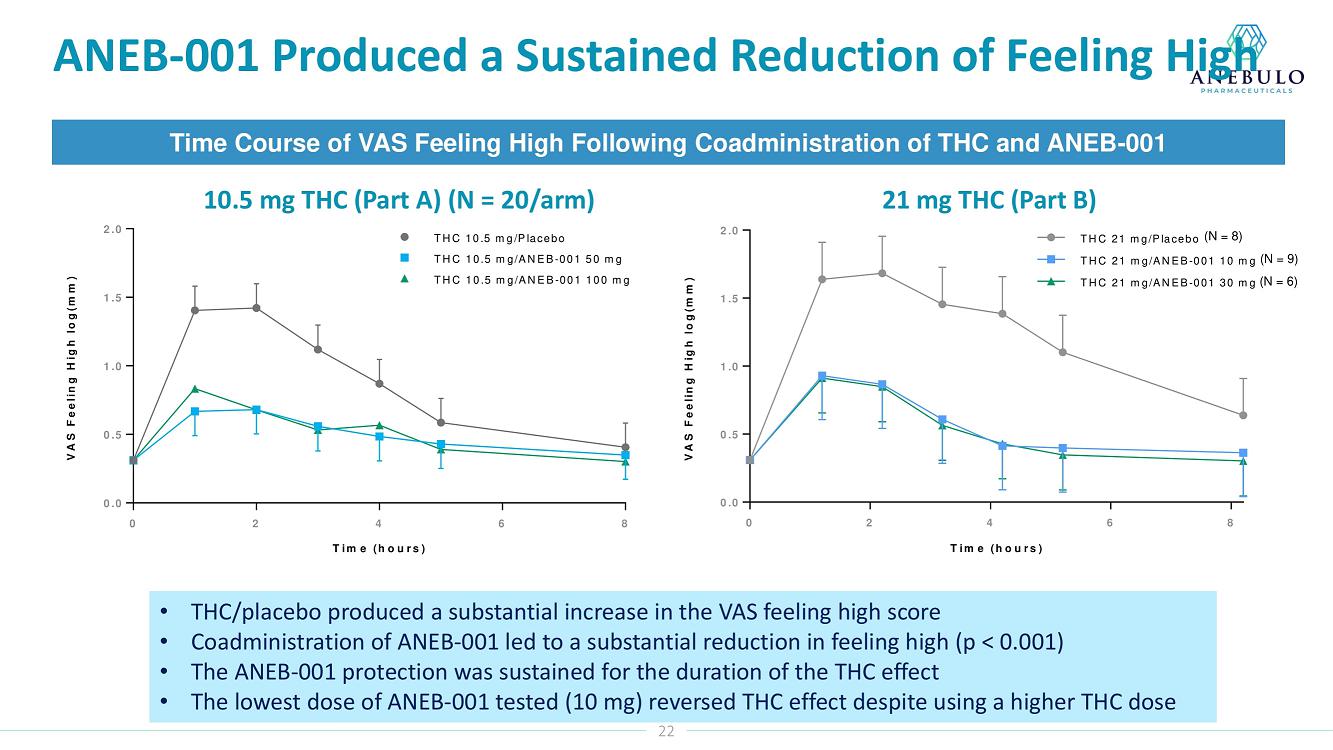
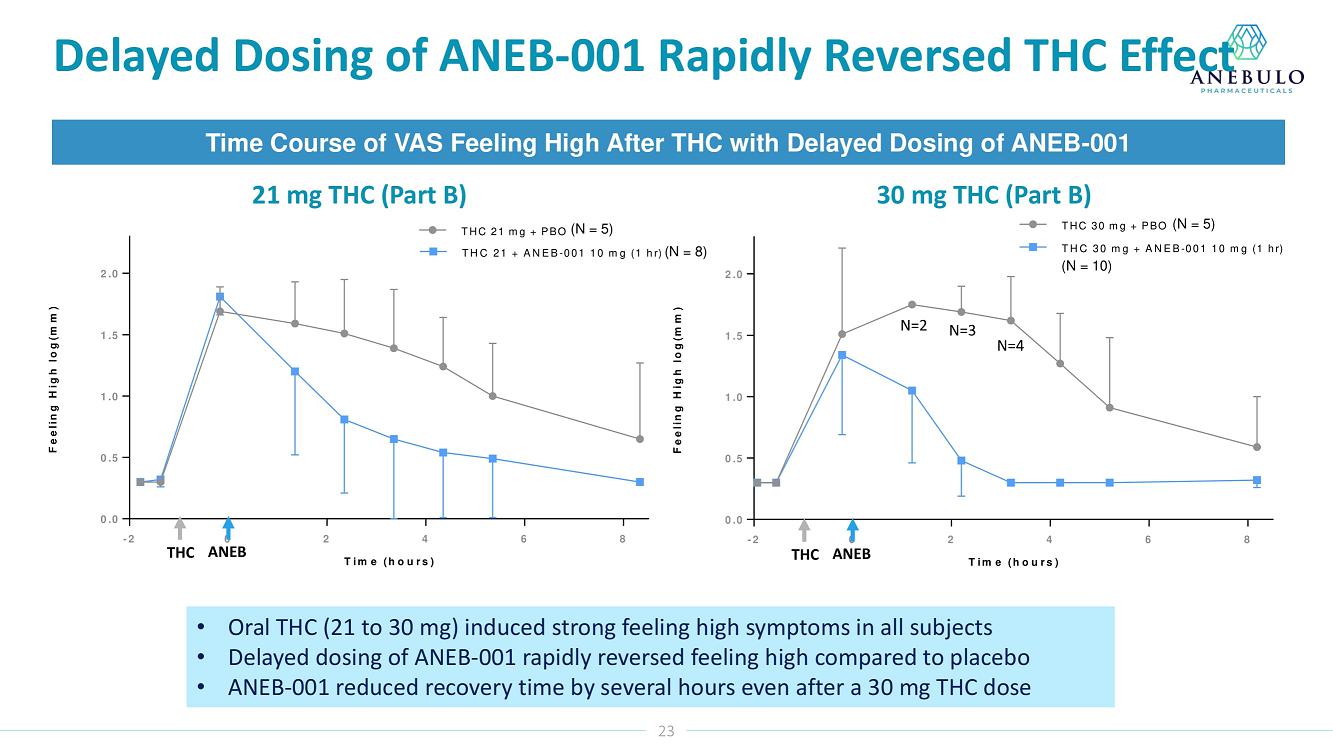
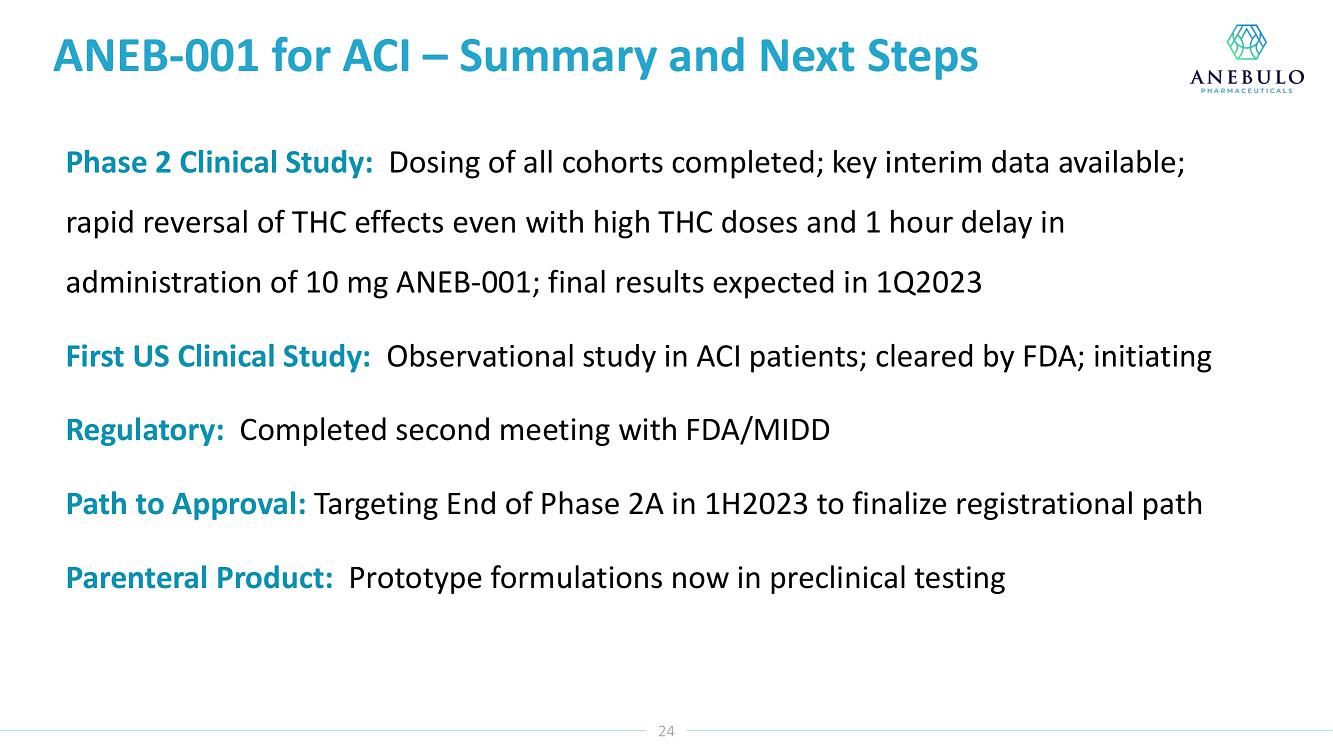
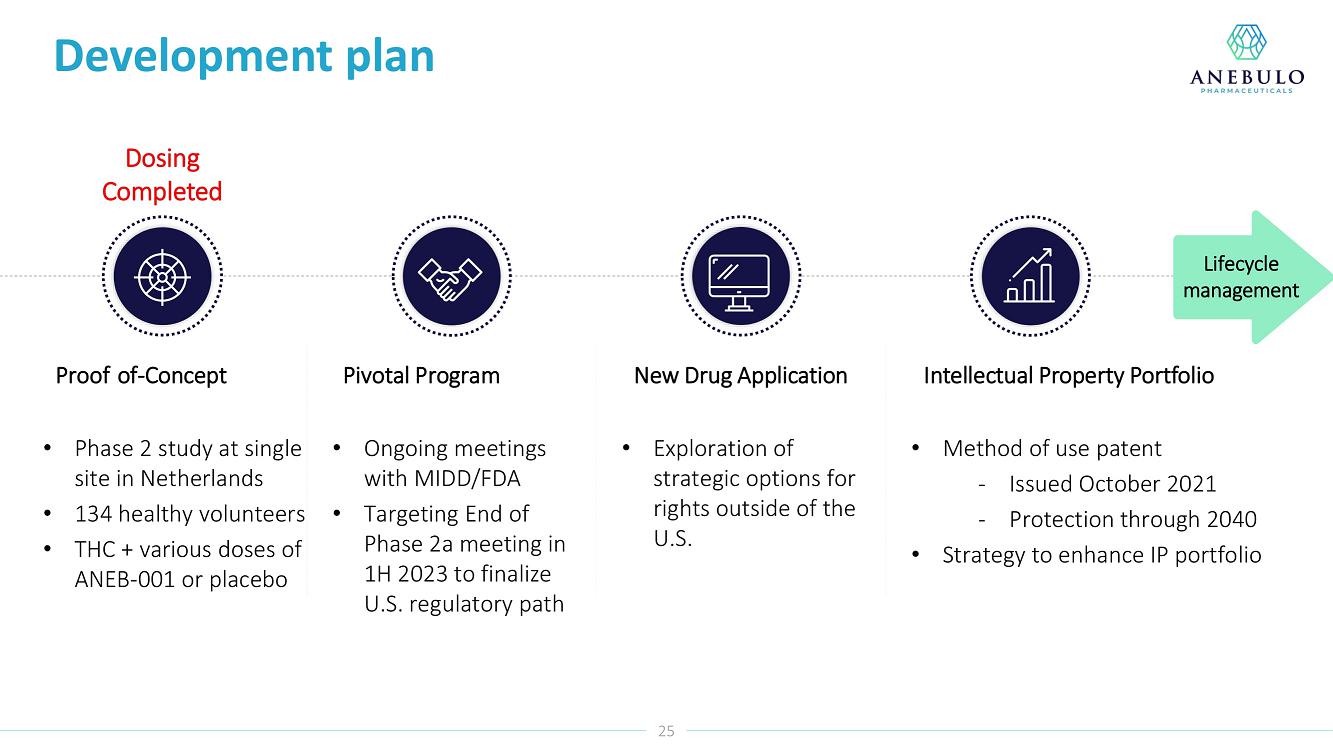
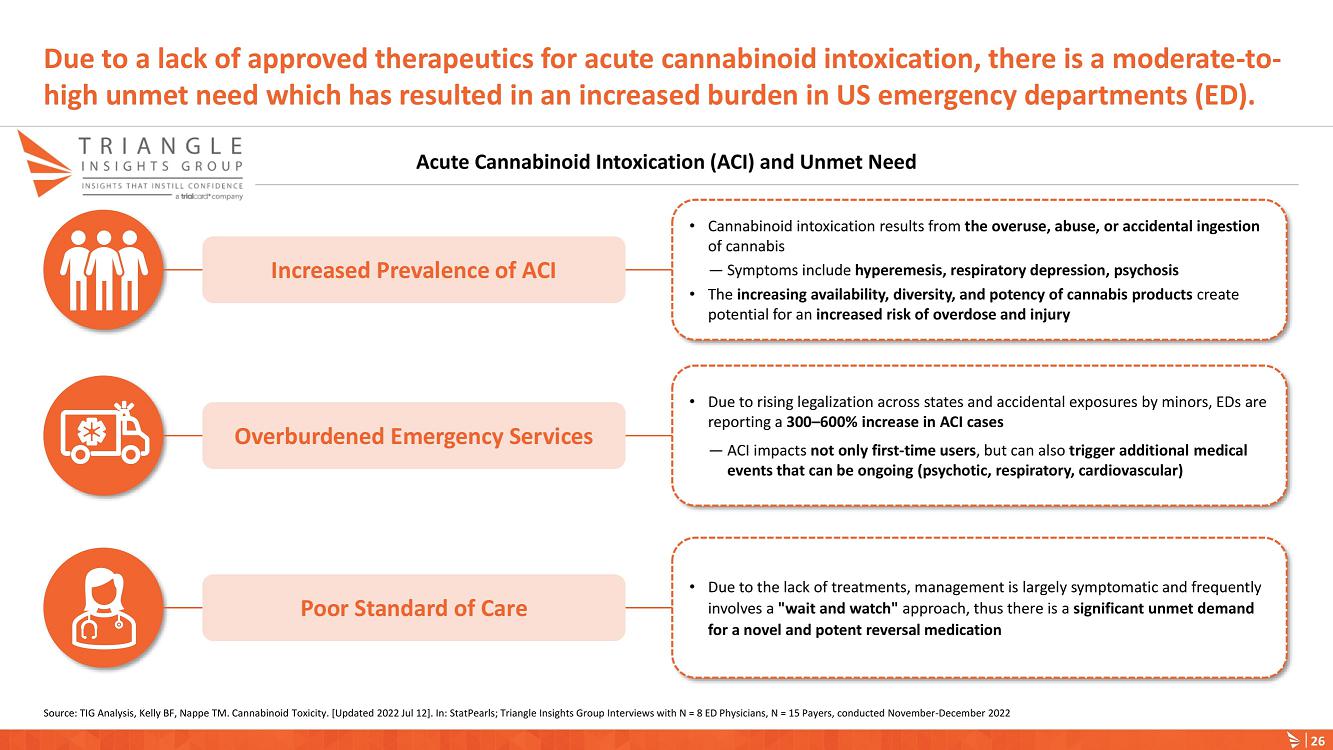
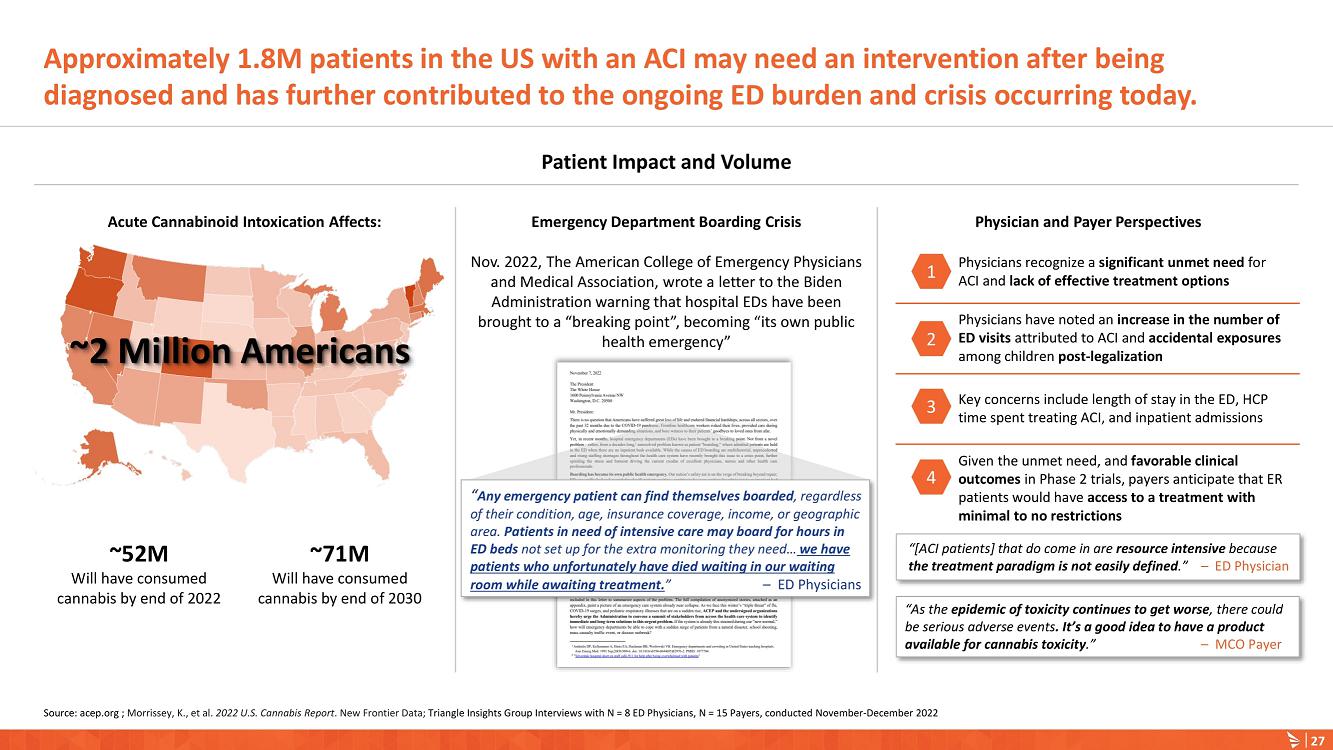
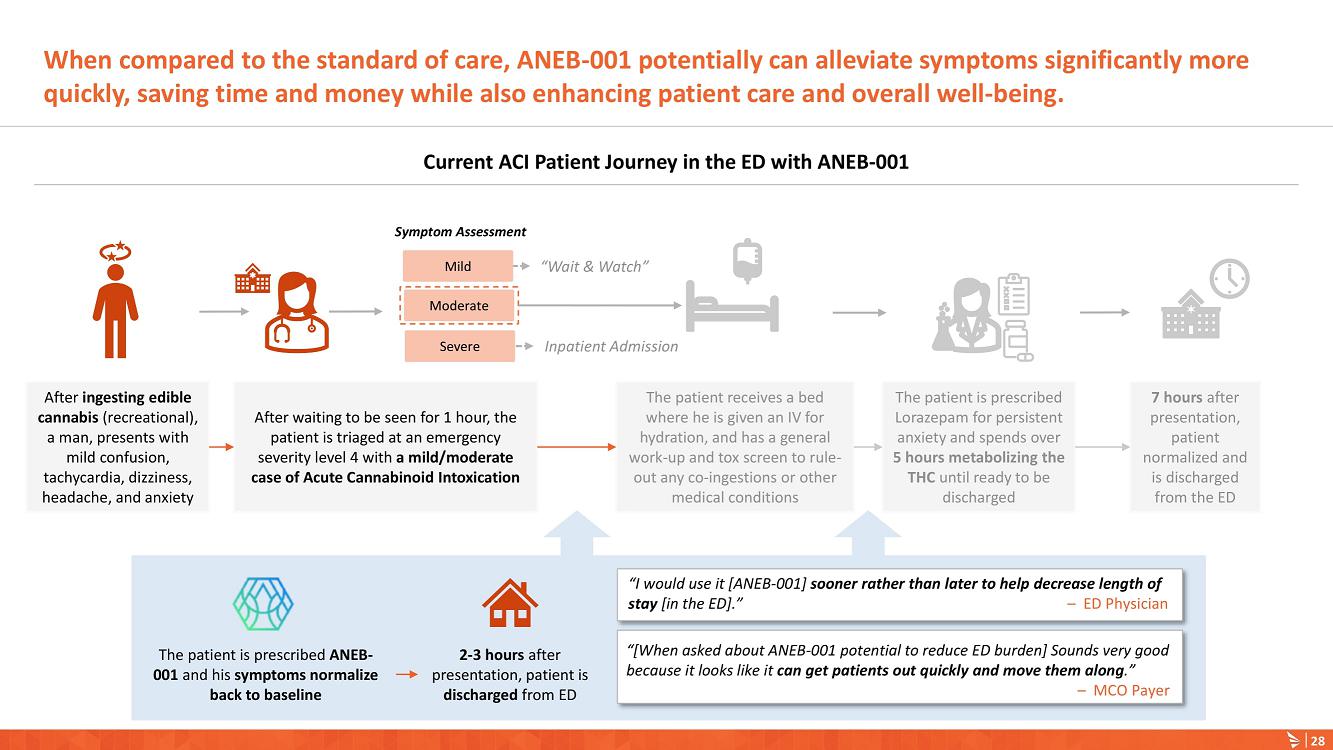
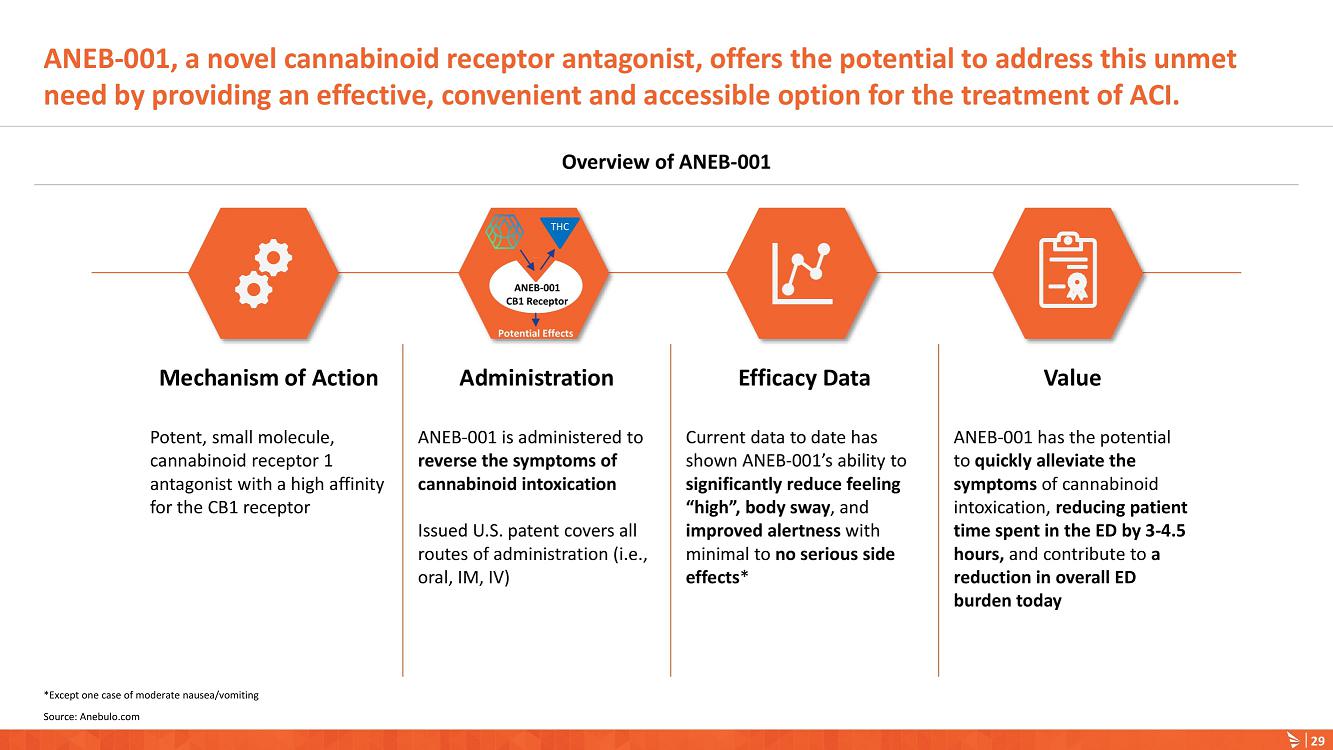
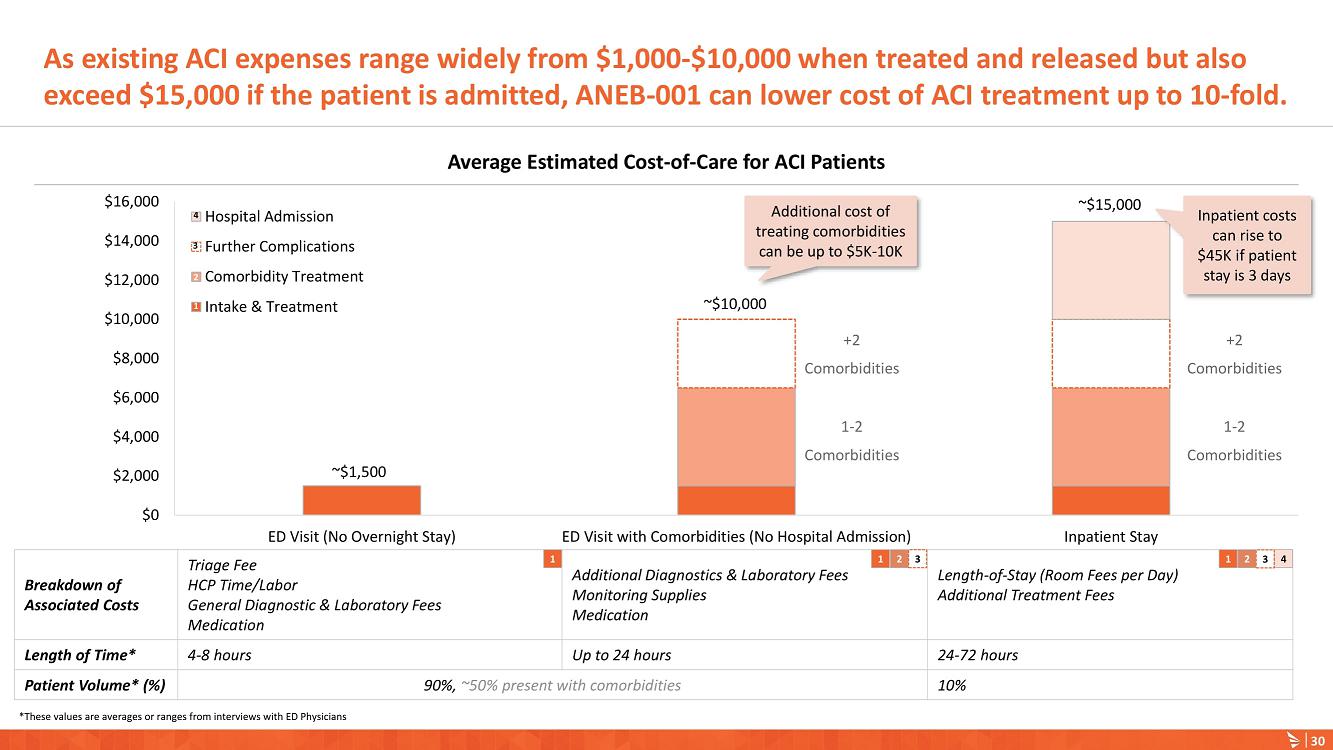
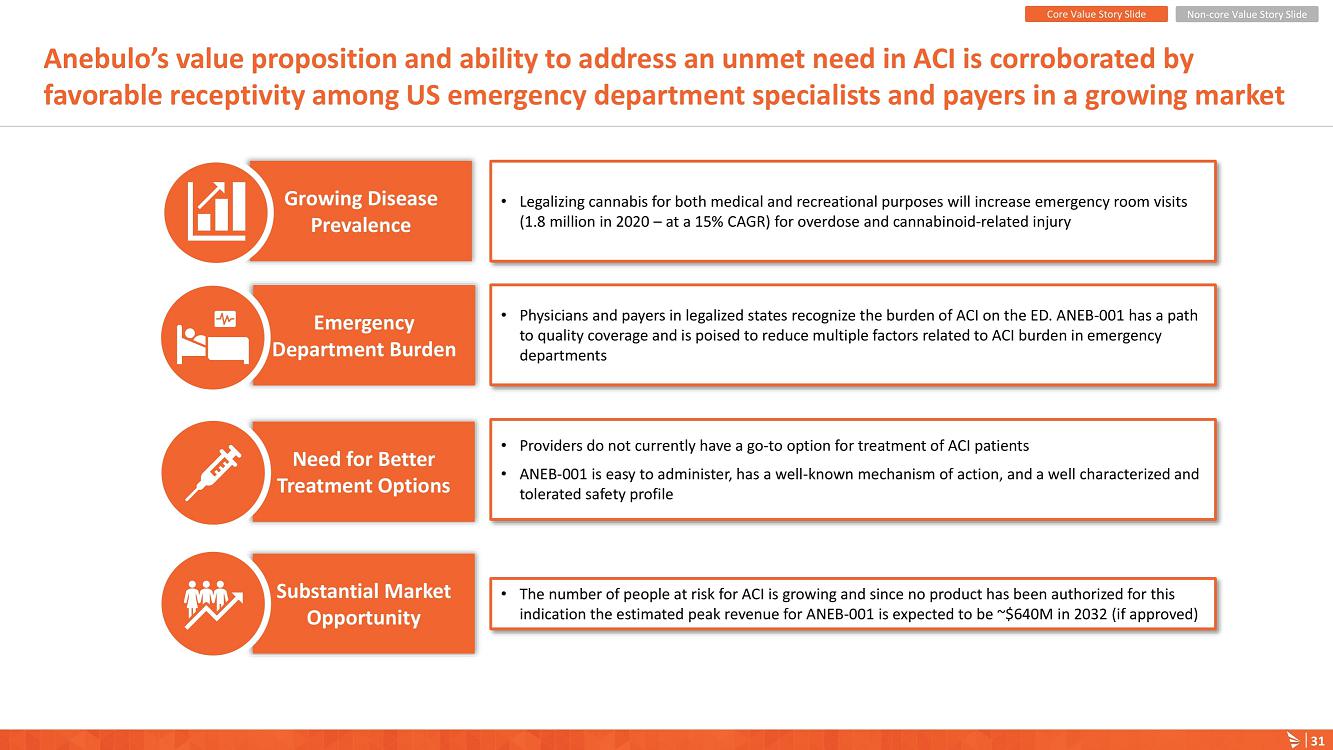


Exhibit 99.2

Anebulo Pharmaceuticals Announces Completion of Dosing and Preliminary Data from Part B of its Phase 2 Study of ANEB-001 for Acute Cannabinoid Intoxication
| ● | Preliminary data showed ANEB-001 reduced effects of a 30 mg dose of THC | |
| ● | Delayed dosing of ANEB-001 rapidly reversed pre-existing THC effects | |
| ● | Full PK, PD, and safety data expected by end of 1Q2023 | |
| ● | Company targeting an End of Phase 2A meeting with FDA in the first half of 2023 |
AUSTIN, Texas (January 9, 2023) – Anebulo Pharmaceuticals, Inc. (Nasdaq: ANEB), a clinical-stage biopharmaceutical company developing novel solutions for people suffering from acute cannabinoid intoxication (“ACI”) and substance addiction (the “Company” or “Anebulo”), today announced completion of dosing in its randomized, double-blind, placebo-controlled Phase 2 clinical trial evaluating ANEB-001 as a potential treatment for ACI in healthy subjects challenged with oral THC. Part B of the Phase 2 trial was an adaptive design that included six cohorts of up to 15 healthy adults to examine different doses of THC and ANEB-001, and the impact of delayed dosing of ANEB-001 or placebo. In total, Parts A and B of the Phase 2 study enrolled 134 healthy subjects.
Data from Part A of the study showed positive protective effects of a single oral dose of 50 or 100 mg ANEB-001 when co-administered with an oral challenge dose of 10.5 mg THC. In Part B of the study, subjects were challenged with substantially higher oral doses of THC (21, 30 or 40 mg) and treated with lower doses of ANEB-001 (10 or 30 mg) or matching placebo. Delayed dosing of ANEB-001 was also examined by introducing a one-hour pause between the THC challenge and treatment with the ANEB-001 or placebo. The final cohort of the study included administration of a high fat meal prior to the THC challenge. Additional analyses await the final unblinded data.
Based on preliminary pharmacodynamic data for Part B of the study, a single low oral dose of ANEB-001 (10 mg) administered 1 hour after THC appeared to rapidly reverse key psychotropic effects of THC doses as high as 30 mg, including reduction in feeling high and improvement in alertness and body sway. ANEB-001 also appeared to reduce the time required for effects to normalize back to baseline. Only 5 subjects were challenged with 40 mg THC due to poor THC tolerability. Final analyses await the unblinded data expected by end of 1Q2023.
“Completing the dosing in this Phase 2 trial represents an important milestone for the company. Preliminary data showed that a single 10 mg dose of ANEB-001 reduced key symptoms of ACI induced with 30 mg of THC,” said Simon Allen, Chief Executive Officer of Anebulo. “Further, we successfully demonstrated rapid reversal of key ACI symptoms even after a one-hour delay between the THC challenge and ANEB-001 dosing. We believe the final data from this study, together with data from our planned observational study in ACI subjects, will provide support for the design of a registrational trial. We anticipate discussing the final data from this study with FDA at an End of Phase 2A meeting in the first half of this year.”
The Phase 2 study was conducted in the Netherlands by the Centre for Human Drug Research (“CHDR”). A total of 134 healthy subjects were enrolled. The safety data for the study are still blinded. There were no serious adverse events. At the 30 mg THC dose, prior to dosing ANEB-001 or placebo, subjects developed mild to moderate THC-related symptoms including euphoria, bradyphrenia, paresthesia, and feeling emotional. After dosing ANEB-001 or placebo, the adverse events considered possibly or probably related to ANEB-001, THC, or placebo were mild except for one case of moderate nausea/vomiting. Administration of a high fat meal delayed the THC effects and the reversal of feeling high by ANEB-001.
About ANEB-001
Our lead product candidate is ANEB-001, a potent, small molecule cannabinoid receptor antagonist, to address the unmet medical need for a specific antidote for ACI. ANEB-001 is an orally bioavailable, readily absorbed treatment candidate that we anticipate will rapidly reverse key symptoms of ACI. ANEB-001 is protected by one issued patent and rights to one patent application covering various methods of use of the compound and delivery systems. We began a Phase 2 proof-of-concept trial for ANEB-001 in December 2021 in the Netherlands and announced positive Phase 2 Part A proof-of-concept topline data on July 5, 2022, positive Part B data on September 26, 2022, and completed dosing of all subjects in mid-December 2022.
About Acute Cannabinoid Intoxication
Symptoms of ACI can include increased somnolence, impaired cognition and perception, disorientation, anxiety, and acute psychosis. According to DSM-5, a diagnosis of cannabinoid intoxication should include recent history of cannabinoid use, clinically considerable behavioral or psychological changes, such as euphoria, impaired judgment and motor skills, which have taken place since cannabinoid exposure.
About the Centre for Human Drug Research
The CHDR is an independent institute that specializes in cutting-edge early-stage clinical drug research. Combining innovative methods and technologies, state-of-the-art facilities, and talented, motivated researchers helps CHDR maximize their clients’ success. In addition, CHDR places the highest priority on their subjects’ comfort and safety, and they play an active role in helping educate the medical and clinical research communities.
About Anebulo Pharmaceuticals, Inc.
Anebulo Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing novel solutions for people suffering from acute cannabinoid intoxication and substance abuse disorder. Its lead product candidate, ANEB-001, has completed dosing in a Phase 2 clinical trial (www.clinicaltrials.gov/ct2/show/NCT05282797) evaluating its utility in reversing the negative effects of acute cannabinoid intoxication. ANEB-001 is a competitive antagonist at the human cannabinoid receptor type 1 (CB1). For further information about Anebulo, please visit www.anebulo.com.
Forward-Looking Statements
Statements contained in this press release that are not statements of historical fact are forward-looking statements as defined in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. In some cases, these forward-looking statements can be identified by words such as “anticipate,” “believe,” “designed,” “expect,” “intend,” “may,” “will,” “should” and other comparable terms. Forward-looking statements include statements regarding Anebulo’s intentions, beliefs, projections, outlook, analyses or current expectations regarding: the Phase 2 Study for ANEB-001 and the design, timing and potential benefits thereof; expected timing for full PK, PD and safety data from Anebulo’s Phase 2 Study of ANEB-001 by the end of the first quarter of 2023; the targeted timing for an End of Phase 2A meeting with the FDA in the first half of 2023; future results that may be implied by prior results; Anebulo’s belief that the data from its Phase 2 Study of ANEB-001, together with data from its planned observational study in ACI subjects, will provide support for the design of a registrational trial; the potential for ANEB-0001 to address an unmet medical need for a specific antidote for ACI; and Anebulo’s expectation that ANEB-001 will rapidly reverse key symptoms of ACI. You are cautioned that any such forward-looking statements are not guarantees of future performance and are subject to a number of risks, uncertainties and assumptions, including, but not limited to: initial and interim results from clinical studies are not necessarily indicative of results that may be observed in the future; clinical trial site challenges that may impact the expected timing of the Company’s ongoing clinical trials, including challenges related to the ongoing COVID-19 pandemic; the timing and success of clinical trials and potential safety and other complications thereof; any negative effects on the Company’s business and product development plans caused by or associated with COVID-19 or geopolitical issues; and Anebulo’s need for additional capital. These and other risks are described under the “Risk Factors” heading of Anebulo’s most recent quarterly report on Form 10-Q filed with the Securities and Exchange Commission on November 10, 2022. All forward-looking statements made in this press release speak only as of the date of this press release and are based on management’s assumptions and estimates as of such date. Except as required by law, Anebulo undertakes no obligation to update or revise forward-looking statements to reflect new information, future events, changed conditions or otherwise after the date of this press release.
CONTACTS:
Anebulo Pharmaceuticals, Inc.
Scott Anderson
Head of Investor Relations and Public Relations
(858) 229-7063
scott@anebulo.com
Rex Merchant
Chief Financial Officer
(512) 598-0931
IR@anebulo.com
# # #