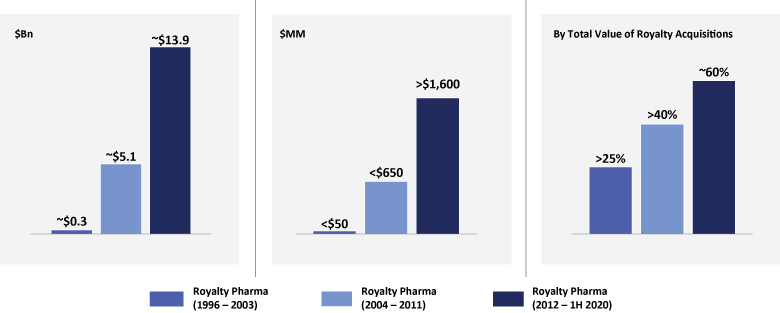
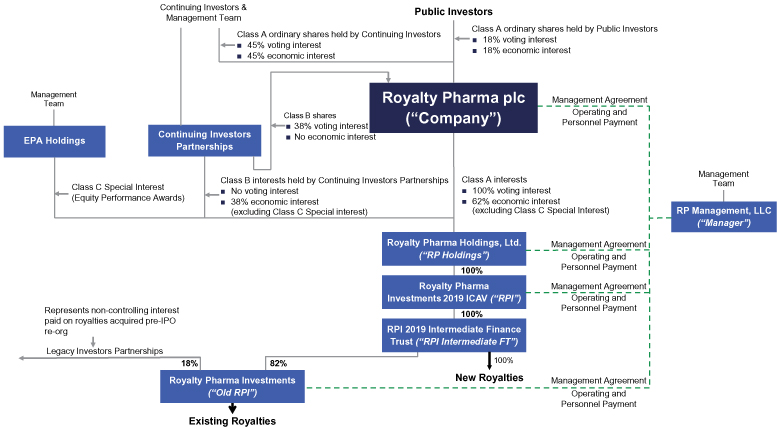
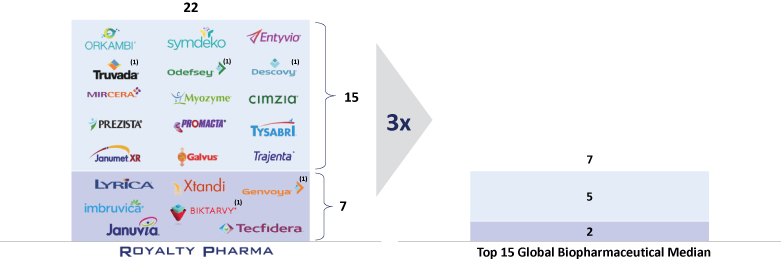
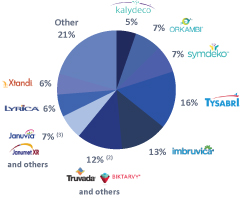
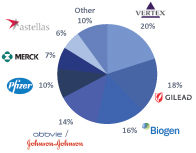
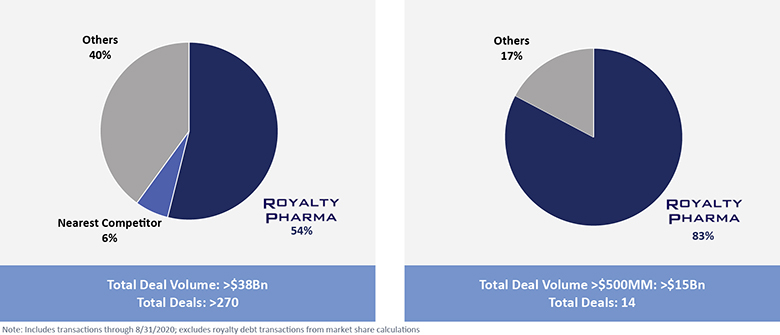
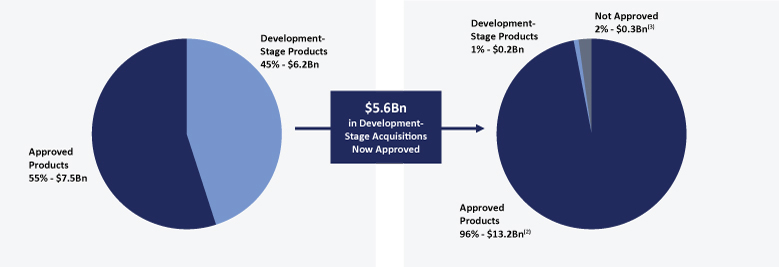
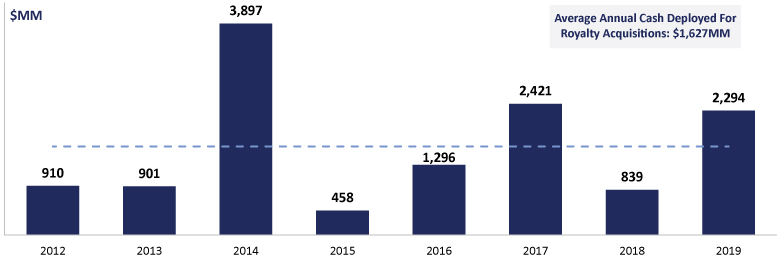
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED OCTOBER 13, 2020
PRELIMINARY PROSPECTUS

17,343,037 Shares
Royalty Pharma plc
Class A Ordinary Shares
The selling shareholders identified in this prospectus are offering all of the Class A ordinary shares being offered under this prospectus. We will not receive any of the proceeds from the sale of Class A ordinary shares in this offering.
Our Class A ordinary are listed on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “RPRX.” The offering price will be determined by negotiations between us and the representatives of the underwriters.
The selling shareholders have granted the underwriters an option to purchase up to 2,601,455 additional shares of Class A ordinary shares at the public offering price less the underwriting discounts and commissions.
Investing in our Class A ordinary shares involves risks. See “Risk Factors” beginning on page 23.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share |
Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to the selling shareholders |
$ | $ | ||||||
| (1) | The underwriters may also exercise their option to purchase up to an additional 2,601,455 Class A ordinary shares from the selling shareholders, at the public offering price, less the underwriting discounts and commissions, for 30 days after the date of this prospectus. In addition, we have agreed to reimburse the underwriters for certain expenses in connection with the offering. Please see “Underwriting” for additional information regarding underwriting compensation. |
The underwriters expect to deliver the shares to purchasers on or about , 2020 through the book-entry facilities of The Depository Trust Company.
| J.P. Morgan | Morgan Stanley | BofA Securities | Goldman Sachs & Co. LLC | Citigroup |
| Cowen | Evercore ISI | Truist Securities | UBS Investment Bank |
, 2020

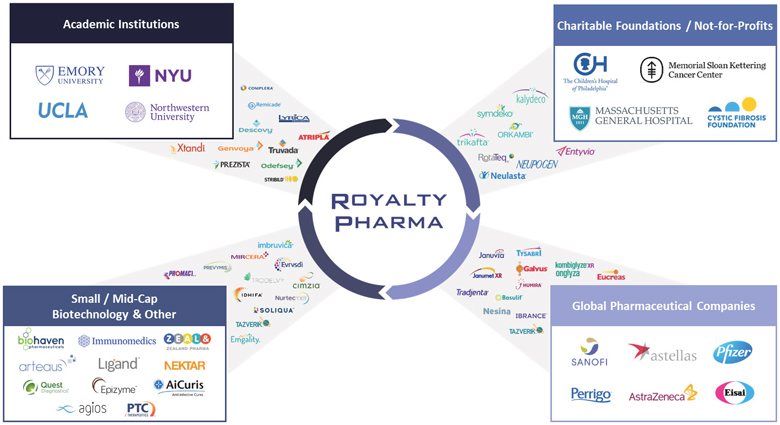

 (2)
(2) 

 (3)
(3)  (4)
(4) 
 (5)
(5)  (6)
(6)